Nestlé has recalled several SMA Infant Formula and Follow-On Formula products as they may contain cereulide toxin.
In a risk statement published yesterday, the company said the "possible presence of cereulide (toxin) makes these products unsafe to consume."
Cereulide is a toxin that is produced by some strains of Bacillus cereus.
Cereulide is highly heat stable and so is unlikely to be destroyed through cooking, using boiling water or when making the infant milk.
If consumed, it can lead to a rapid onset of symptoms, which include nausea, vomiting and abdominal cramps.
Although there have not been any confirmed reports of illness associated with the consumption of the products concerned, Nestlé has said that, out of an abundance of caution, it has decided to perform this voluntary recall in line with its strict product quality and safety protocols.
The company has confirmed that the recall is global, with affected products sold in several European countries, including France, Germany, Austria, Denmark, Italy and Sweden.
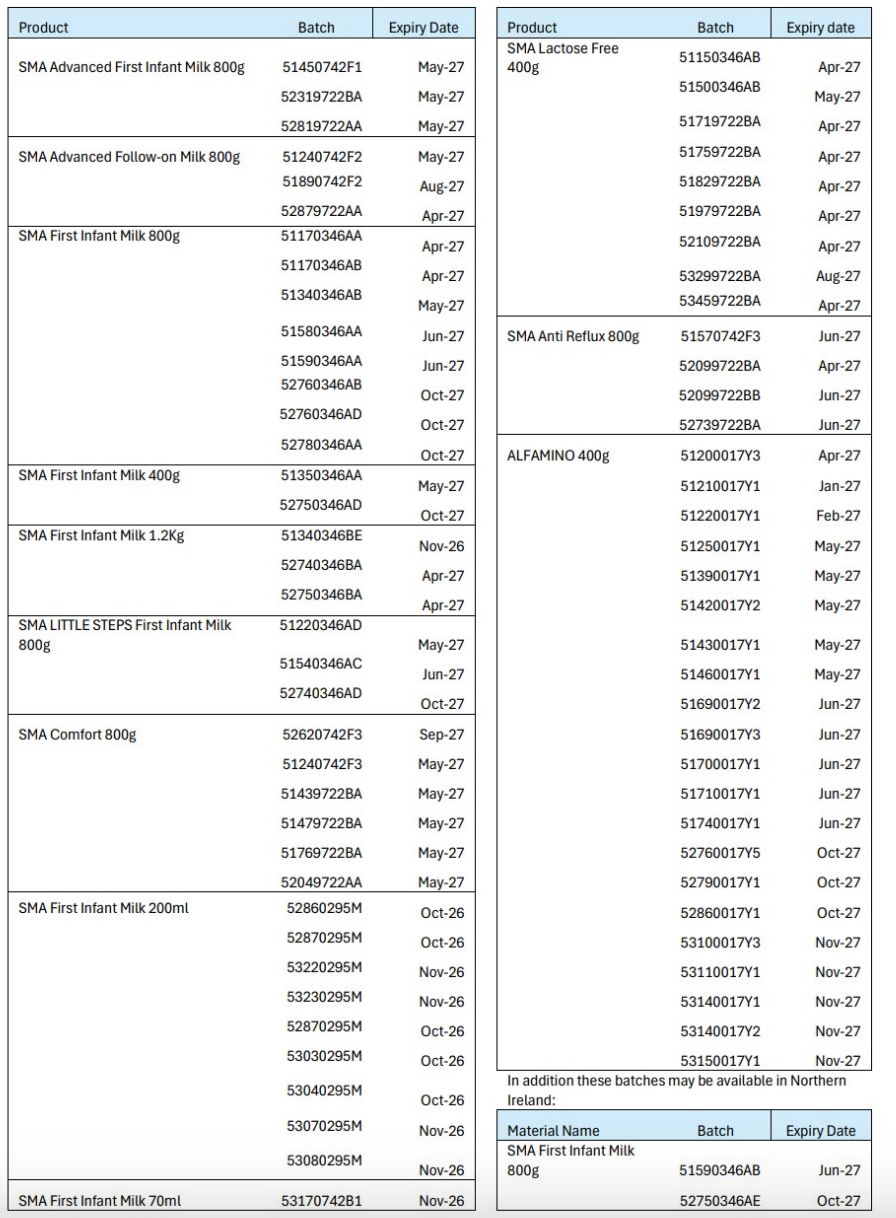
The specific batch numbers of the products affected are listed on the FSA and Nestlé websites.
Batch numbers can be found on the base of the tin or box for powdered formulas, or on the base of the outer box and on the container for ready-made formulas.
Point of sale notices will be displayed in all retail stores that are selling these products. These notices explain to customers why the products are being recalled and tell them what to do if they have bought the products.