µSMIN Plus is a dietary supplement ingredient in the form of granules that are dissolved easily when ingested to optimise conversion of diosmin into the bioactive aglycone (diosmetin) in the gastrointestinal tract (figure 1).
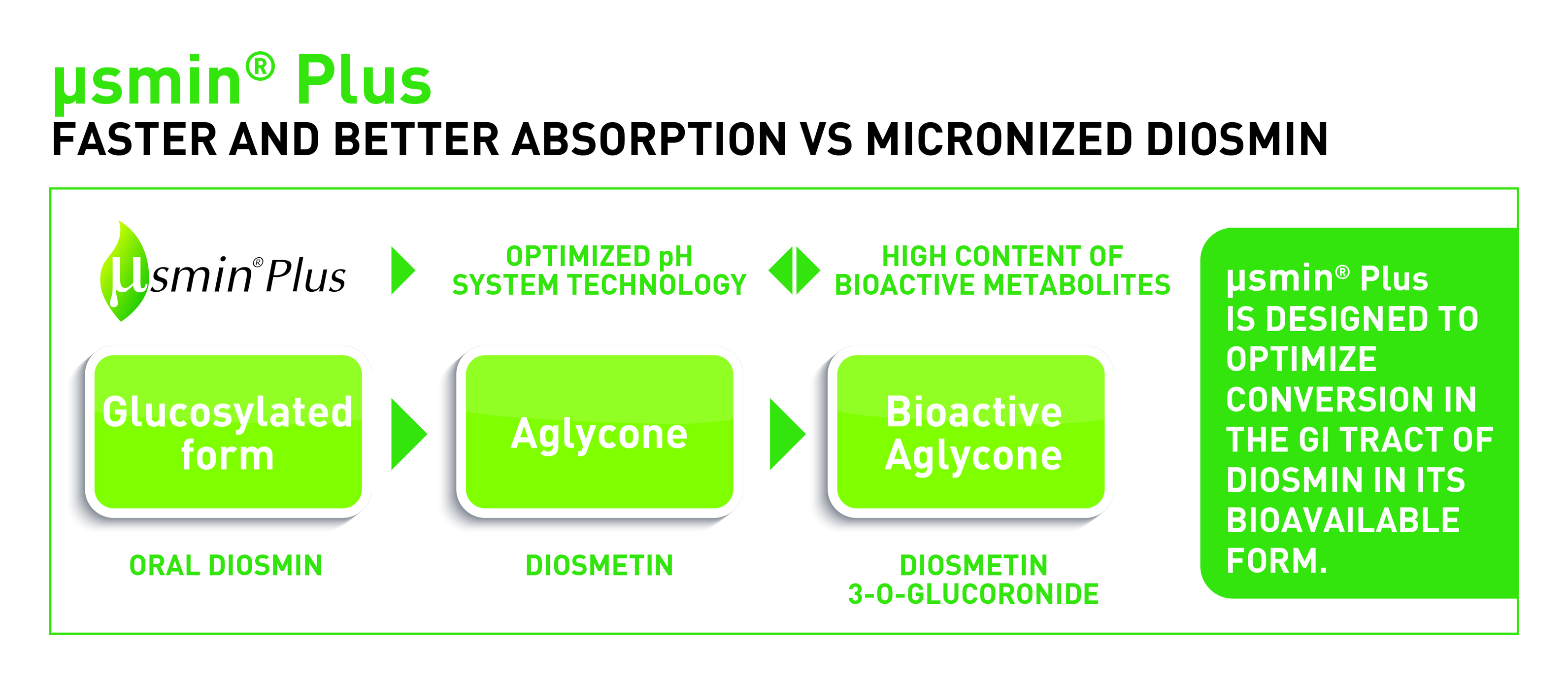
Figure 1.
Diosmin (3’,5,7-trihydrozy-4’-mehtoxyflavone-7-rutinside) is a flavonoid commonly present in most plants and fruits, especially citrus fruits.1 It can be also manufactured by extracting hesperidin from citrus rinds through iodine-assisted oxidation. Its molecule includes a sugar moiety (rutinoside disaccharides) linked to the aglycone diosmetin (figure 2).
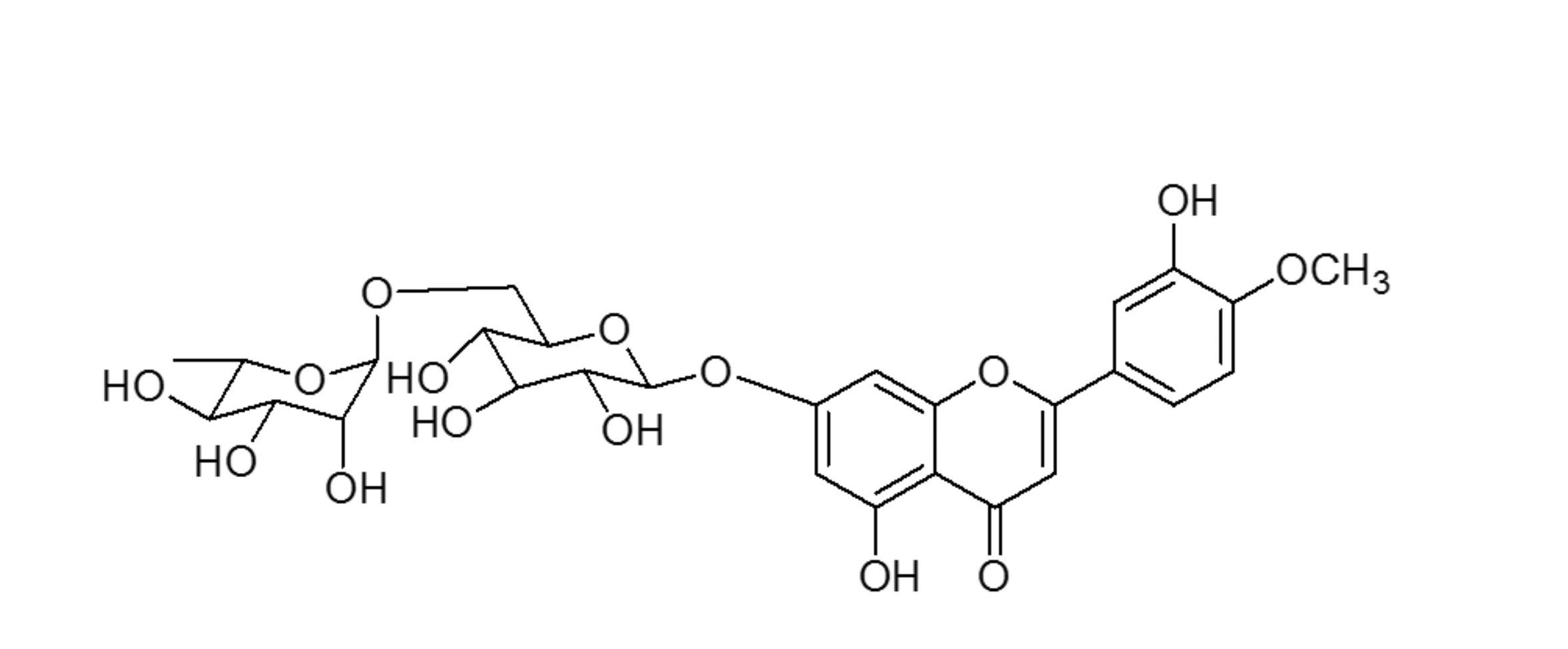
Figure 2. Chemical structure of diosmin
Clinically, diosmin has been used for more than 30 years in Europe for its phlebotonic and vascular protecting actions in pharmaceutical and nutritional products.2 Diosmin is primarily used in the management of vascular disorders including chronic vascular insufficiency (CVI), hemorrhoids, lymphedema, and varicose veins.3
Despite the potential health benefits of diosmin, its phenolic nature renders it polar and poorly water-soluble resulting in poor dissolution. Poor dissolution is responsible for its poor bioavailability in humans following oral administration. Over the past decade, several experimental attempts have been carried out to enhance the bioavailability of flavonoids including diosmin. Even though the micronisation can be considered a consolidated technology to improve bioavailability of poorly soluble substances such as diosmin, further increases in oral bioavailability are required to achieve optimal therapeutic efficacy.
Based on a 2015 pharmacokinetic study in rats demonstrating the superior oral bioavailability of µSMIN Plus compared to micronised diosmin,4 a human study was completed. The double-blind, cross-over study in 16 healthy volunteers, compared plasma diosmetin (a diosmin metabolite) levels following oral administration of either 500 mg of μSMIN Plus or a 500 mg of a standard micronised diosmin5. Plasma concentrations of diosmetin were significantly higher following oral administration of μSMIN Plus compared to the standard micronised diosmin. The relative bioavailability was 9.4 times greater for μSMIN Plus than the micronised diosmin (figure 3).
Combined, these results suggest that μSMIN Plus has superior bioavailability and may provide the health benefits of diosmin as significantly lower doses compared to conventional micronised diosmin.
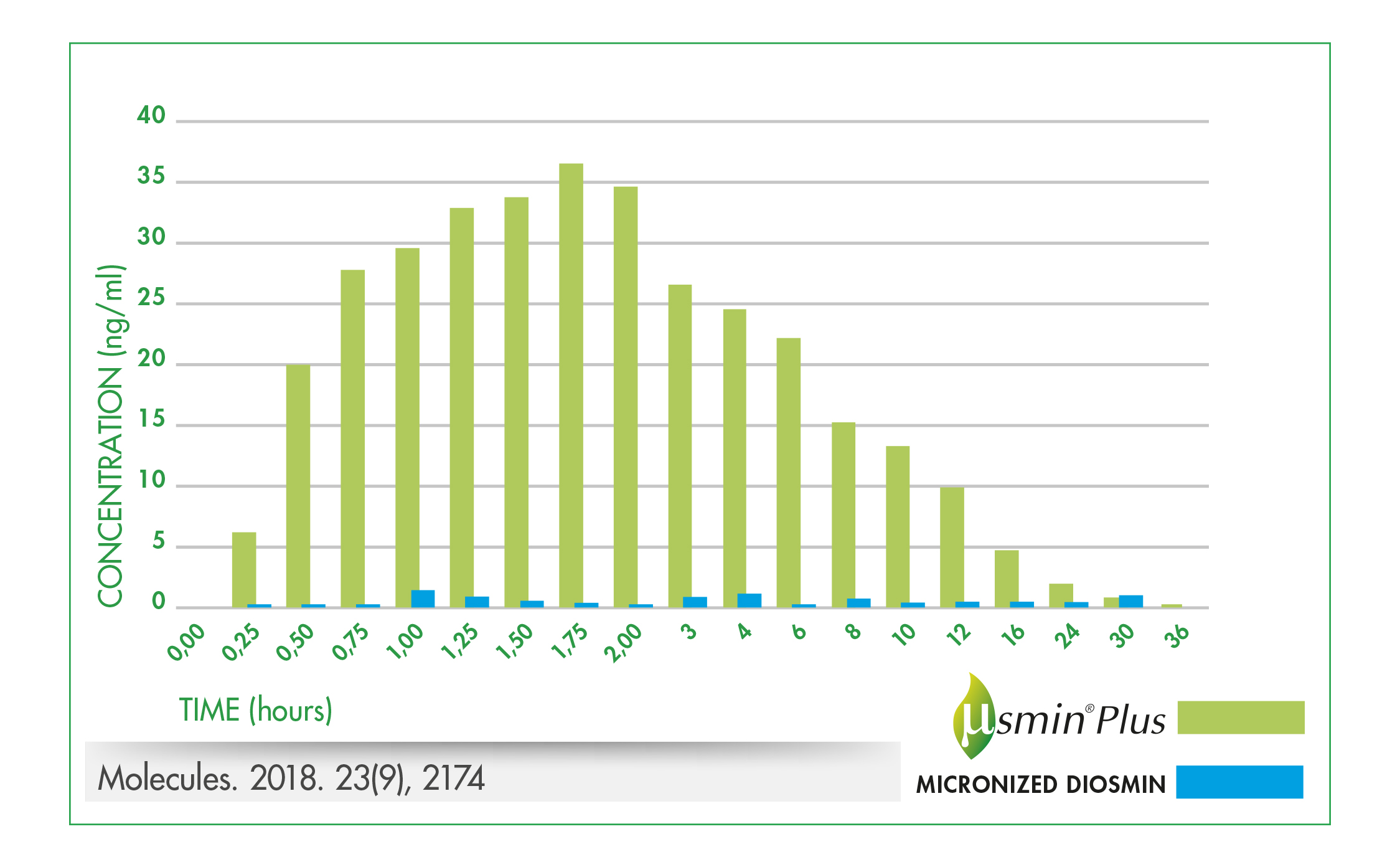
Figure 3.
Giellepi SPA is pleased to announce the start of a human efficacy trial with μSMIN Plus in Autumn of 2019.6 Designed to study the efficacy of oral μSMIN Plus (450 mg of micronised diosmin) in chronic venous insufficiency, the eight-week, randomised, double-blind, placebo-controlled trial will enroll approximately 68 subjects. Results are expected in the middle of 2020.6
To learn more about μSMIN Plus from Giellepi SPA, visit FT-Pharma USA at Booth #4270 at SupplySide West.
References
1. Kanaze FI, Gabrieli C, Kokkalou E, et al. Simultaneous reversed-phase high-performance liquid chromatographic method for determination of diosmin, hesperidin and naringin in different citrus fruit juices and pharmaceutical formulations. J Pharmacol Biomed Anal 2003;33:243-9.
2. Bush R, Comerota A, Meissner M, et al. Recommendations for the medical management of chronic venous disease: The role or micronized purified flavonoid fraction (MPFF). Phlebology 2017;32:3-19.
3. Diosmin Monograph. Altern Med Rev 2004;9:308-11.
4. Russo R, Mancinelli A, Ciccone M, et al. Pharmacokinetic profile of µSMIN® Plus, a new micronized diosmin formulation, after oral administration in rats. Nat Prod Commun 2015; 10:1569-72.
5. Russo R, Chandradhara D, De Tommasi N. Comparative bioavailability of two diosmin formulations after oral administration to healthy volunteers. Molecules 2018;23, 2174; doi: 10.3390/molecules23092174.
6. www.ClinicalTrials.gov
