Even before COVID-19 struck, more and more companies were moving away from complex in-house tablet coating formulations to a leaner production model using ready prepared formulations. This trend continues to accelerate owing to heightened concerns about inherent risks in supply chains.
Colorcon has been at the forefront of helping companies to convert to fully formulated film coating systems. With seven strategic manufacturing locations around the world, Colorcon provides the uninterrupted supply of innovative solutions based on the one-step Opadry Complete Film Coating System, which provides significant benefits compared with a do-it-yourself (DIY) approach.
One-step film coatings for solid oral dose forms, originally developed by Colorcon, have been available for more than 50 years and their use is standard practice for most pharmaceutical manufacturing companies.
Compared with in-house coating, ready made formulations offer substantial benefits, including reduced inventory management, better coating efficiency, increased manufacturing capacity, batch to batch consistency and reduced overall costs.
Companies that still rely on in-house coating formulation have long recognised the benefits of new, leaner production models; but, in many cases, fear of disruption has resulted in existing systems remaining in place. The current pandemic has brought about a reassessment as minimising risk to supply chains has become an overriding priority … and fully formulated coating systems are now moving from on-hold to active implementation (Figure 1).
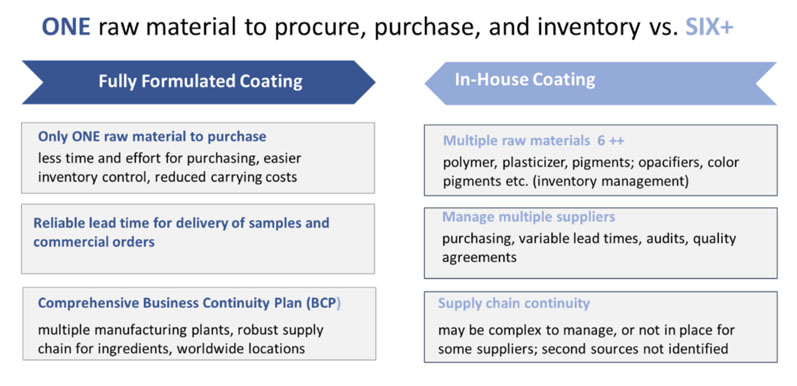
Figure 1: A comparison of fully formulated versus in-house coating processes
The traditional approach to producing film coatings in-house involves many steps. First, raw materials must be sourced; then quality control procedures must be undertaken for all delivered materials. These materials are likely to come from several vendors, each of whom may need to be audited and approved.
The raw materials must be stored with associated inventory management costs before undergoing a series of preproduction and production stages. As ingredients are added individually, there is a risk of error owing to the selection of the wrong ingredient.
The addition of each material takes both time and effort and must be done safely and precisely to achieve a homogenous suspension; high shear mixing is often required to ensure the correct dispersion of pigments.
Dispensing your coating materials also introduces cleaning considerations and the risk of cross-contamination. Creating a stable, consistent coating is not a simple process, especially for non-specialists, and achieving colour consistency and uniformity presents significant challenges.
Colour is contingent on the particle size distribution of pigments and any batch-to-batch inconsistency will result in final coating colour variation.
The alternative — a fully formulated coating — is much less risky, as it involves just one material from one supplier and a single quality check. This means less documentation, quick and easy handling, shorter production times, consistent quality and, crucially, minimal supply chain risk.
A reputable and reliable coating vendor will perform the quality audits of its suppliers, ensure alternative sources of supply in case of unforeseen events and provide regulatory support.
Keeping colour consistent
Colour uniformity is one of the most difficult attributes to achieve, but Colorcon’s instrumental and visual colour difference tests ensure a consistent colour every time. Instrumental tests confirm that the hydrated powder meets the formula standard colour and is measurable using a reflectance spectrophotometer.
Appearance testing ensures that the colour, odour and homogeneity of the powder blend conforms to the item specification. Visual colour difference tests of the actual film compare past batch history information to ensure batch-to-batch consistency and confirm the instrument-derived colour data.

Adopting a lean process
Colorcon offers customised, one-step film coating systems that combine the basic ingredients — polymer, plasticiser and pigment — in a dry concentrate. The Opadry range of film coating systems provides best-in-class formulations that ensure quality and consistency for a wide range of functional applications and colour requirements.
Although the benefit of these coatings is often considered in terms of productivity and cost saving, the quality assurances and risk mitigation associated with development, production and performance should not be understated. Rigorous formulation, material and supply chain controls, alongside operational processes and testing requirements, guarantee exceptional and repeatable coating performance. Key benefits include the following:
- elimination of separate inventories (polymer, plasticiser and pigment)
- simple preparation and ease of use
- optimised equipment usage and resource allocation
- quality control savings
- batch-to-batch colour consistency
- reliable supply chain for pharmaceutical manufacturers worldwide
- expert technical and regulatory support.
To ensure that coating formulations developed by Colorcon are both innovative and robust in use, the company has adopted four quality by design (QbD) principles to support the range of Opadry coatings and ensure both consistent colour and reliable coating performance with each batch.
- Design of experiment (DoE) to enhance coating formulations and process optimisation. From development to launch, all Opadry systems undergo a rigorous process to assess, implement and ensure optimum functionality.
- Process validation ensures that every new Opadry system meets required quality specifications, at every scale of manufacture, at any of Colorcon’s seven interchangeable manufacturing plants around the world.
- Stability testing according to ICH guidelines ensures consistent and stable product appearance, colour, dispersion properties and coating performance during the specified shelf-life of the product.
- A unique master formulation serves as a global reference to create all subsequent batch records, no matter where they are produced around the world.
Optimising the final finish
The drawbacks for any tablet manufacturer of an in-house film coating mainly relate to quality issues: raw materials, appearance, colour variation, stability or batch-to-batch consistency. These issues can result in crucial reproducibility difficulties during scale-up and commercial manufacturing.
A fully formulated Opadry film coating system will help to address these issues, but the final finish will only be as good as the quality and control of the coating process itself. So, it’s also important to understand and manage the critical process parameters (CPPs) of the coating process: dispersion spray rate; spray distribution; process temperatures; airflow; coating solids concentration; pan loading and pan speed.
Evaluating the supplier network
The supplier network is crucial, and service providers should be evaluated on their capabilities, use of appropriate good manufacturing practices, business continuity planning (BCP) and be routinely monitored for ongoing performance. At Colorcon, there are industry guidelines in place to ensure that finished product testing operates within a certified quality management system.
Colorcon operations meet the Joint IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients and ISO9001 certification. Ensuring that a supplier has a robust BCP will help to minimise risk.
With more than 50 years of experience, Colorcon not only upholds an unparalleled pedigree of developing innovative coating solutions, but also provides extensive technical knowledge to ensure coating equipment and processes are fully optimised.
Colorcon has seven film coating manufacturing plants located strategically around the world, which meet the needs of both global and local markets. All facilities operate with the same raw materials, the same equipment and the same processes; plus, a considerable amount of work has been done to validate the interchangeability of products from each of the sites.
Superior support: when and where it’s needed
Colorcon has 22 technical support laboratories worldwide to help customers get the best from their coating process. Companies can run trials in Colorcon’s coating laboratories, seek advice and get insight on troubleshooting.
They can also participate in an educational programme through the Colorcon Coating School to address theoretical aspects of film coating, access hands-on experience with laboratory and production scale coating equipment, explore troubleshooting and gain insight into the latest developments of coating formulations.
There has never been a more pressing case to move towards lean, right-first-time coating systems to ensure high-quality products and to mitigate risks in these troubled times.




