Ginsenosides are triterpene saponins. Most ginsenosides are composed of a dammarane skeleton (17 carbons in a four-ring structure) with various sugar moieties (such as glucose, rhamnose, xylose and arabinose) attached to the C-3 and C-20 positions. More than 30 ginsenosides have been identified and classified into two categories:
- 20(S)-protopanaxadiol (PPD) (Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, Rs1)
- 20(S)-protopanaxatriol (PPT) (Re, Rf, Rg1, Rg2, Rh1).
The difference between PPTs and PPDs is the presence of a carboxyl group at the C-6 position in PPDs. Moreover, several rare ginsenosides, such as the ocotillol saponin F11 (24-R-pseudoginsenoside) and the pentacyclic oleanane saponin Ro (3,28-o-bisdesmoside) have also been identified. Asian ginseng (Panax ginseng) is commonly known as the true ginseng.
In this article, HPTLC methods suitable for the analysis of ginsenosides are presented, using CAMAG equipment, Merck thin layer chromatography (TLC) plates, analytical standards and extract reference materials. The extract reference materials are manufactured by HWI Analytik and exclusively distributed by Merck Sigma-Aldrich.
The detection of ginsenosides in the HPTLC fingerprint of different Panax species (roots and root extracts) is obtained by following the HPTLC method of the Ph. Eur. monograph (1), USP DSC 2015 monograph (2) and the method of the International Association for the Advancement of HPTLC (3) by comparing the RF values and colours of reference substances and matching zones in the root extract.1–3
Depending on the regulation followed, one of these three methods of identification can be used.
Recommended CAMAG devices: automatic TLC sampler (ATS), an automatic developing chamber (ADC), a TLC visualiser, a chromatogram immersion device, a TLC plate heater and visionCATS.
Derivatisation reagent: anisaldehyde (1) or sulphuric acid (2,3).
Sample: 0.015 g/mL extract (HWI extract) in 70% methanol (1).
Standards: Standard solutions of ginsenosides were prepared in a concentration of 0.5 mg/mL in methanol (Tables I and II).
Chromatography following USP <203>4
Stationary phase: HPTLC Si 60 F254 20 x 10 cm (Merck).
Sample application: (1,3) 4 µL of each test solution and 2 µL of the standards were applied as 8 mm bands (8 mm from the lower edge, 20 mm from the left edge); (2) 4 µL each of test solution and 4 µL of the standards were applied as 8 mm bands (8 mm from the lower edge, 20 mm from the left edge).
Developing solvent: (1) ethyl acetate/water/butanol (25:50:100 v/v/v), upper layer; (2) dichloromethane/ethanol water (70:45:6.5 v/v/v); (3) chloroform/ethyl acetate/methanol/water (15:40:22:9 v/v/v/v).
Development: (1) In the ADC with an unsaturated chamber and after conditioning at 33% relative humidity for 10 min using a saturated solution of magnesium chloride; (2,3) development is performed with an ADC, saturated for 20 minutes with the developing solvent (filter paper). Prior to development, the plate is conditioned for 10 min to a relative humidity of 33% (with a saturated solution of MgCl2).
Developing distance: (1,2) 70 mm (from lower edge), (3) 80 mm (from lower edge).
Plate drying: 5 min in a stream of cold air.
Derivatisation: (1) The plate is immersed (immersion speed: 3 s, immersion time: 0 s) into anisaldehyde reagent (mixture of 0.5 mL of p-anisaldehyde, 10 mL of glacial acetic acid, 85 mL of methanol and 5 mL of sulphuric acid) with the chromatogram immersion device and heated for 5 min at 105 °C.
(2,3) The plate is immersed (immersion speed: 3 s, immersion time: 0 s) into sulphuric acid reagent (10% in methanol) with the chromatogram immersion device and heated for 5 min at 105 °C.
Evaluation: Documentation under white light (1–3) and UV 366 nm (2,3) after derivatisation with the TLC visualiser.
Results and discussion
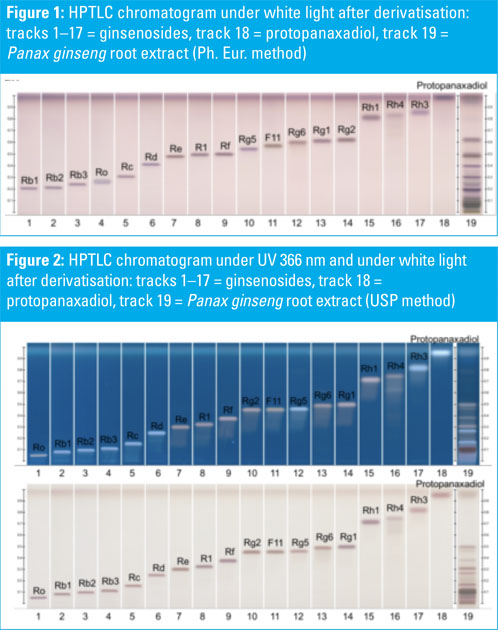
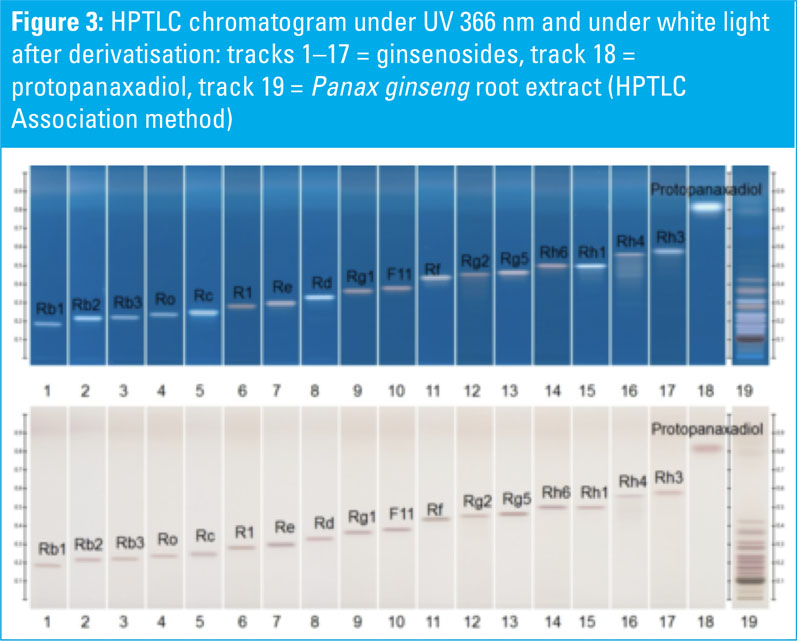
HPTLC chromatograms of ginsenoside standards and a Panax ginseng root extract are shown in Figures 1–3. HPTLC chromatograms of plants containing ginsenosides (different Panax species) are shown in Figures 4–6.

P. ginseng, P. quinquefolium, P. notoginseng, P. japonicus and P. vietnamensis roots and root extracts were collected and analysed. The P. ginseng root extract was used as a botanical reference material to identify Asian ginseng (the ginsenoside Rf should be present and F11 absent).
All shown methodologies are suitable for the detection of ginsenosides in different Panax species. Ginsenoside Rf is unique to Asian ginseng whereas F11 is found exclusively in American ginseng.
Thus, the Rf/F11 ratio can be used as a phytochemical marker to distinguish American ginseng from Asian ginseng. In the used botanical reference material (Panax ginseng root extract, article no: 0511-50-01) the presence of Rf and the absence of F11 could be confirmed with all methods and it is therefore suitable for the identification of Asian ginseng.
Methods one (Ph. Eur.) and two (USP) benefit from being official monographs in the respective pharmacopoeias, whereas method three is an alternative procedure derived from the HPTLC Association.
The method is improved for the separation of Rf and F11 to better distinguish American and Asian ginseng. The derivatisation with sulphuric acid reagent (methods two and three) leads to different coloured zones under UV 366 nm, which is useful for identification.
References
- European Directorate for the Quality of Medicines and HealthCare, “Ginseng Root,” monograph in Ph. Eur. 8.0, 01/2008:2383 (Strasbourg, France).
- United States Pharmacopeial Convention, “Tienchi Ginseng Root and Rhizome Dry Extract,” monograph in USP 40-NF35 (Rockville, MD, USA, 2017).
- HPTLC Association, “HPTLC Identification Method for Panax ginseng,” www.hptlc-association.org.
- United States Pharmacopeial Convention, “<203> High-Performance Thin-Layer Chromatography Procedure for Identification of Articles of Botanical Origin,” in USP 39-NF34. (Rockville, MD, USA, 2016).




