Bonerge is set to unveil its second-generation Amuctive Akkermansia muciniphila.
This will come ahead of its showcase at the upcoming SupplySide Global 2025 ingredient exhibition.
Akkermansia muciniphila is a human intestinal symbiont that contributes to the maintenance of a healthy gut barrier.
It is involved in regulating immunity and also limits the onset of inflammation.
The launch marks a strategic pivot for the industry, moving beyond counting bacterial colonies to ensuring the potency of a key active protein, Amuc_1100.
The development addresses a common consumer frustration that, despite the recognised benefits of Akkermansia for metabolic and gut health, many existing supplements fail to deliver results.
Bonerge's innovation targets the root cause: the discrepancy between high bacterial counts and low biological activity.
From "live vs pasteurised" to "active vs inactive"
Traditional Akkermansia supplements often revolved around the debate of live versus pasteurised bacteria.
While live Akkermansia faces challenges such as shelf stability, sensitivity to antibiotics and potential safety concerns in some populations, research indicates that properly pasteurised Akkermansia could be more effective.
Bonerge's solution builds on this finding, emphasising that the true source of Akkermansia's benefits is not the bacterium itself, but the robust Amuc_1100 protein present on its membrane.
This protein remains active after pasteurisation and acts as the primary effector molecule, responsible for supporting immune function, regulating glucose and lipid metabolism and protecting the gut barrier.
Without sufficient Amuc_1100, high bacterial counts are effectively "empty shells," offering little therapeutic value.
Second-gen Amuctive Akkermansia: quantifying activity
The core innovation of the second-generation Amuctive Akkermansia lies in its proprietary production and quality control processes, specifically designed to maximise and verify Amuc_1100 content.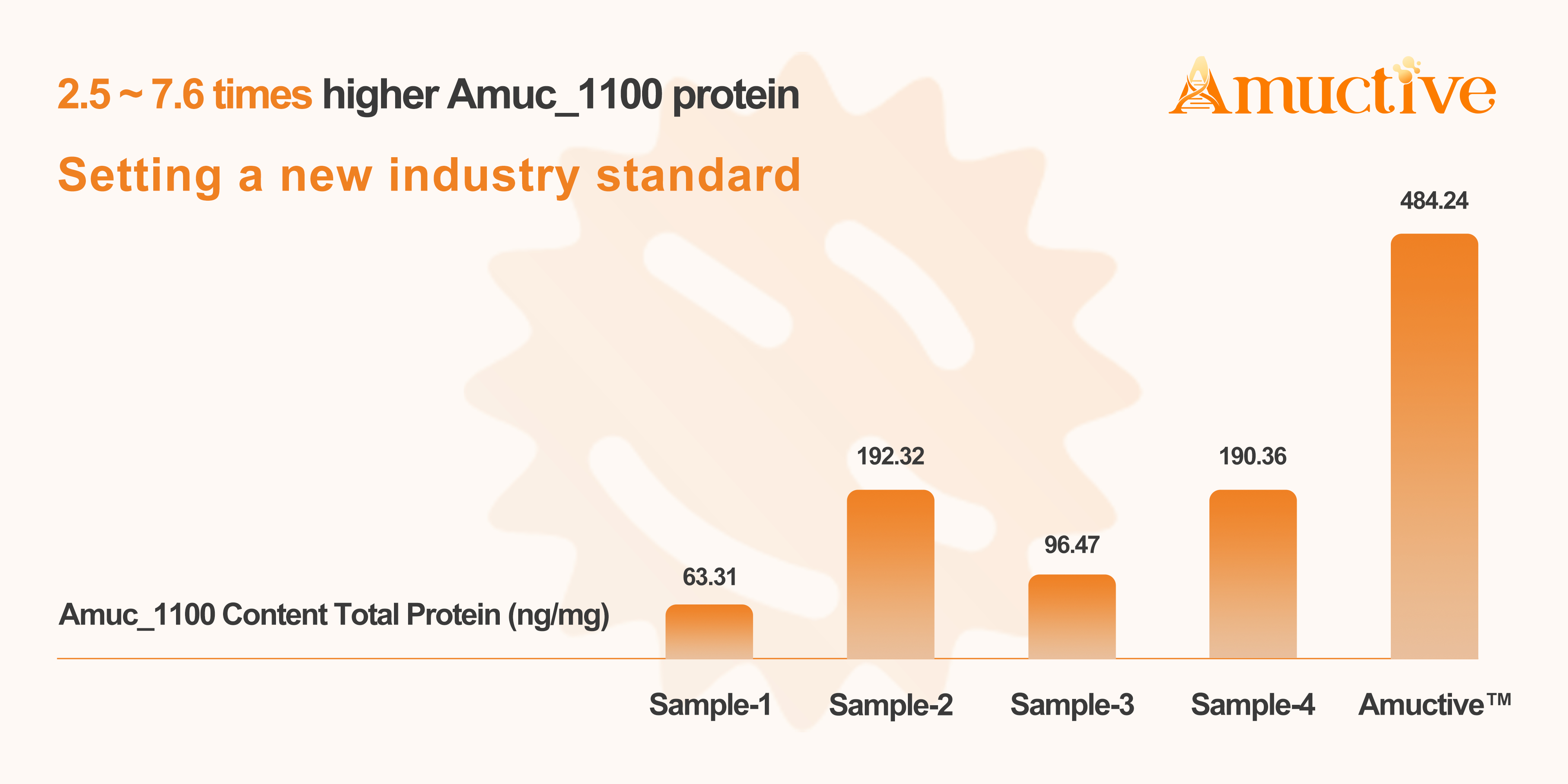
Through a patented fermentation and inactivation process, Bonerge achieves an Amuc_1100 protein content verified to be 2.5 to 7.6 times higher than conventional Akkermansia ingredients.
The company has developed a proprietary detection technology to accurately quantify Amuc_1100 levels throughout production.
This ensures consistent activity from batch to batch, a critical step towards reliable efficacy.
The ingredient holds US SA-GRAS certification and is backed by multiple patents and internal studies demonstrating benefits for metabolic health and ageing.
SSW showcase and broader portfolio
At SupplySide Global 2025, booth #4331-1, Bonerge will highlight this next-generation Akkermansia as part of its broader portfolio of high-quality anti-ageing ingredients.
The exhibit will also feature other innovative compounds, including Fisetin, Urolithin A, S-Equol, L-Ergothioneine and PQQ, underscoring the company's focus on science-backed, clinically relevant solutions for longevity.
In addition, Bonerge will sponsor a featured session at the Product Development Theater titled “Discover the Next Generation of Akkermansia muciniphila.”
Presented by nutrition expert Jim Roufs, the talk will explore why certain Akkermansia supplements show limited efficacy and how Amuctive achieves measurable results through its patented process and elevated Amuc_1100 protein content.
The session will take place on October 29th, 2025, from 3:00-3:20 pm at booth #8076.
Bonerge emphasises that its commitment to safety and efficacy is embodied in the second-gen Amuctive Akkermansia, transforming an intangible concept, activity, into a measurable and controllable quality parameter.
This launch is poised to set a new standard for probiotic and postbiotic supplements, shifting the industry's focus from quantity to proven potency.
