Background
Gastroesophageal reflux disease (GERD), defined as troublesome symptoms or mucosal damage resulting from the reflux of gastric content into the esophagus, is one of the most common gastrointestinal tract disorders with an estimated prevalence higher than 21% in Europe1, 28% in USA2 and up to 33% in Asia and Middle Est2.
The most common symptoms associated with GERD are heartburn and regurgitation; extra esophageal symptoms, including dysphagia, chronic cough, globus sensation, odynophagia, hoarseness, wheezing and nausea, may also occur.
There are two main forms of GERD called erosive reflux disease (ERD) and non-erosive reflux disease (NERD); the latter subcategory represents up to 70% of patients with typical reflux symptoms. Notably, NERD patients have a significant lower response rate to proton pump inhibitor (PPI) therapy3.
A clinical trial recently published in the European Journal of Gastroenterology and Hepatology4 was aimed to assess the efficacy of RefluG™ Isorepair in patients with NERD. (Ribaldone et al. 2020).
Methodology
The efficacy and tolerability of RefluG™ Isorepair has been assessed in a randomized, double blind placebo-controlled trial including 40 NERD patients.
Patients were treated for 2 consecutive weeks with either placebo or RefluG™ Isorepair (3 sachets per day after main meals or before bed).
The primary outcome was to evaluate the efficacy of treatments in terms of the proportion of patients with a significant remission of NERD symptoms (heartburn and regurgitation), defined as a total symptom score (TSS) reduction of at least 3 points assessed according to the 5-point Likert scale.
Secondary outcomes included reduction of Heartburn Severity Index, improvement of Health-Related Quality of life (SF-36 Questionnaire), safety and tolerability.
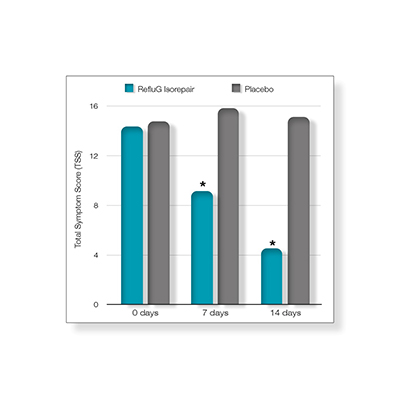
Findings
At the end of treatment, a 3-point reduction in the Total Symptoms Score (TSS) was achieved by 95% of patients treated with RefluG Isorepair, while only 20% of patients treated with placebo showed a 3-point reduction (P < 0.0001; fig. 1).
In patients taking RefluG™ Isorepair, TSS was decreased 68% at the end of the treatment vs baseline.
Heartburn severity index was found to be statistically lower among patients treated with the investigational product compared to those in the placebo group both at Day 7 (P < 0.0001) and at Day 14 (P < 0.0001). Unlike the placebo group, Quality of life was improved at the end of treatment in patients taking the investigational product.
Overall, RefluG™ Isorepair resulted safe and well tolerated; no adverse effects were observed in either of the study groups.
Conclusion
In NERD patients, RefluG™ Isorepair led to a statistically significant improvement in symptoms such as heartburn and regurgitation compared to placebo. Such symptomatic benefit is likely related to the strengthening of the esophageal barrier against the damage induced by gastric contents flowed back into the esophagus during reflux episodes.
Given its mechanism of action, this product may be used alone or in combination with the current pharmacological interventions for the management of GERD.
REFLUG™ ISOREPAIR in a nutshell
REFLUG™ ISOREPAIR is a unique patent-pending combination in liquid sachets registered as Medical Device Class IIA in Europe. Thanks to its protective effect, the product favors the natural healing of the mucosa reducing the unpleasant symptoms associated with acid reflux.
References
- Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018;67:430–400.
- El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014; 63(6):871-80.
- Hershcovici T & Fass R. Nonerosive Reflux Disease (NERD) - An Update. J Neurogastroenterol Motil. 2010; 16(1):8-21.
- Ribaldone et al. A randomized, double-blind, placebo-controlled pilot study to evaluate the efficacy and tolerability of a novel oral bioadhesive formulation for the treatment of non-erosive reflux disease-related symptoms. European Journal of Gastroenterology and Hepatology. August 2020, https://pubmed.ncbi.nlm.nih.gov/32804843/




