Giellepi's µSMIN Plus is a dietary supplement ingredient in the form of granules that dissolve easily when ingested to optimise conversion of diosmin into the bioactive aglycone (diosmetin) in the gastrointestinal tract.
Diosmin is a naturally occurring flavonoid present in citrus fruits and other plants belonging to the Rutaceae family. It can be also manufactured by extracting hesperidin from citrus rinds through iodine-assisted oxidation. Its molecule includes a sugar moiety (rutinoside disaccharides) linked to the aglycone diosmetin (figure 2).
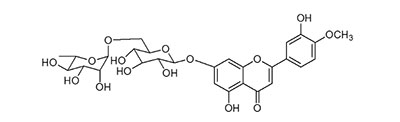
Figure 2.
Diosmin has been used for more than 30 years in Europe for its phlebotonic and vascular protecting actions [1] in the treatment of vascular disorders including chronic vascular insufficiency (CVI), hemorrhoids, lymphedema, and varicose veins. It has also been investigating for other potential clinical applications such as intestinal inflammatory conditions (i.e. ulcerative colitis), ocular disease and diabetes [2-4].
Despite the wide therapeutic potential, diosmin is poorly water-soluble and, consequently, it is scarcely absorbed by the intestinal mucosa following oral intake. Over the past decade, several experimental attempts have been carried out to enhance the bioavailability of flavonoids including diosmin. Even though the micronisation can be considered a consolidated technology to improve bioavailability of poorly soluble substances such as diosmin, further increases in oral bioavailability are required to achieve optimal therapeutic efficacy.
Recently, the improved pharmacokinetic profile of µSMIN Plus has been confirmed both by a preclinical study and by a pharmacokinetic double-blind, two-period, crossover study in healthy volunteers [5-6]. The study compared μSMIN Plus (test product) to unformulated micronised diosmin (reference), in 16 healthy volunteers following oral administration. The tested formulation showed higher plasmatic concentrations of diosmetin in comparison to those obtained after the administration of unformulated micronised diosmin and the relative bioavailability was 9-fold greater for μSMIN Plus than micronised diosmin (figure 3).
According to the exciting results from these trials, μSMIN Plus may provide a variety of health benefits starting from lower therapeutic doses offering a superior alternative to conventional micronised diosmin.
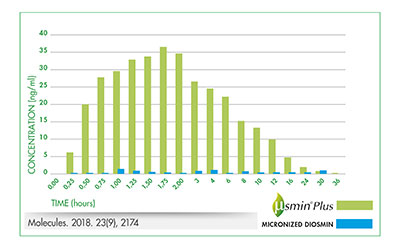
Figure 3.
References
1. Monograph. Diosmin. Altern Med Rev. 9(3), 308-11 (2004).
2. A.S. Shalkami, M. Hassan, A.G. Bakr, "Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis," Hum Exp Toxicol. 37(1), 78-86 (2018).
3. W.Y. Liu, S.S. Liou, T.Y. Hong, I.M Liu, "The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions", Molecules. 22(12)(2017).
4. Clinical Trial Registry; NCT number: NCT02361437.
5. R.Russo, et al., "Profile of µSMIN Plus, a new Micronised Diosmin Formulation, after Oral Administration in Rats" Nat Prod Commun,10(9),1569-72 (2015).
6. R. Russo, D. Chandradhara, N. De Tommasi, "Comparative Bioavailability of Two Diosmin Formulations after Oral Administration to Healthy Volunteers", Molecules 23(9) (2018).
Visit Giellepi SpA at booth #2680




