One such method is a more modern and advanced multiplex microarray technology that offers improved testing accuracy and efficiency.
Consumer packaged goods such as nutraceuticals, pharmaceuticals and supplements require testing for microbial/bacterial by-products to ensure consumer safety.
In the US, for example, manufacturers of these products must test for non-life-threatening class indicator organisms, total aerobic bacteria (TAB), bile-tolerant Gram negative (BTGN) bacteria, total Enterobacteriaceae/coliform (ENT/COL) and total yeast and mould (TY&M).
Traditionally, testing for the total count or enumeration of these class indicator organisms has relied on microbial plate cultures.
Although Petri dish technology has been used as a testing method for more than 130 years, these plate cultures produce results with high degrees of variation and the process is both time- and cost-inefficient.
By comparison, cost-effective multiplexed microarray technology is a more functional solution that yields highly accurate results in less time.

Milan Patel
This technology has benefits beyond the nutraceutical, pharmaceutical and supplements markets as it can also be used for cosmetics, shelf-stable consumer products and food safety testing as well.
Standard methods lack accuracy and efficiency
Plate cultures have a degree of variation (up to 0.5 log) on either side of a threshold, limiting their accuracy and, therefore, their ability to protect consumer safety.
For example, a manufacturer of dietary supplements will need to prove that the TAB equals 1000 colony forming units (CFU) or lower.
But, with a 0.5 log variation of 500, the plate culture result provides a considerable discrepancy and could potentially validate a sample with up to 1500 CFU — half again as much as is allowed.
Culture-based results also take time to develop: approximately 2 days for bacterial testing and up to 6 for fungal organisms. This can result in significant opportunity costs along the supply chain.
Manufacturers spend valuable resources holding products during the waiting period, could potentially fail to achieve their full output potential and run the risk of a recall if testing yields unacceptable class indicator organism levels.
Microarray technology offers a better approach
Multiplexed microarray testing offers a better approach. PathogenDx’s QuantX DNA microarray technology measures the microbial load in a sample while providing discrimination of the organism content relative to testing standards.
The technology is unique in that it offers transparency regarding which species are driving the microbial load.
QuantX combines multiplexed polymerase chain reaction (PCR) with specific single-stranded DNA probes — fixed to a glass microscope slide — to quantify bacterial and fungal pathogens in less than 8 hours without the need for sample enrichment.
Rather than running tests on four separate Petri dishes for each of the class indicator organisms, tests are run on the same array and can check for both live and dead micro-organisms.
The assay utilises internal standards and specific probes to quantify the total CFU in a sample containing bacteria and/or fungi. This breakthrough in molecular diagnostics allows multiplexed detection at a sensitivity and specificity unmatched by similar platforms.
The array contains probes that are specific for bacterial and fungal indicator classes (TAB, BTGN, ENT/COL and TY&M), fragment-specific probes and positive and negative controls.
Each probe is printed in triplicate in each well, allowing for both reproducibility and statistical significance. Once the array is scanned, the QuantX algorithm converts the relative fluorescence units (RFU) into CFU per gram.
The graphs below illustrate a case study showing how QuantX results correlate with Petri dish testing (Figure 1).
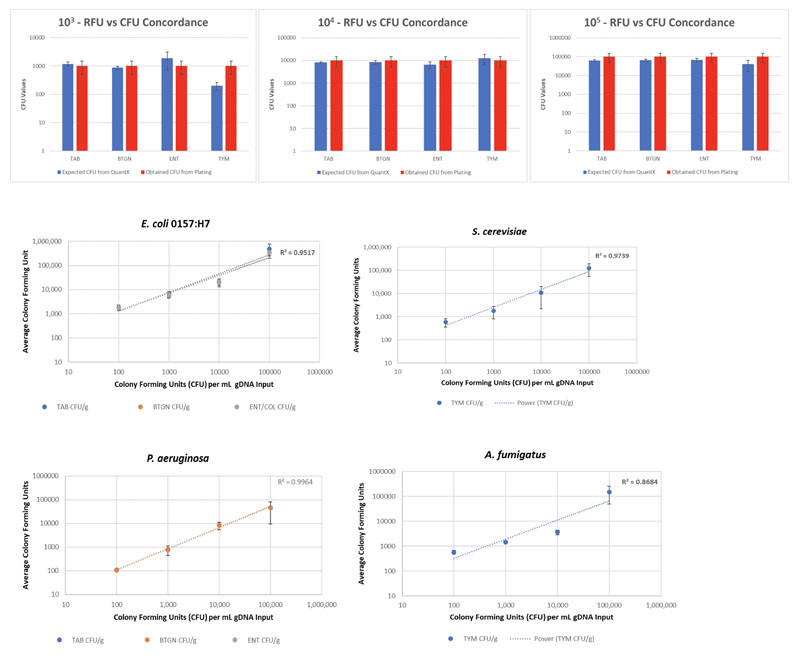
Figure 1: Expected (QuantX) and obtained (plating) CFU results for four test organisms
To demonstrate assay linearity, the study examined two bacterial organisms (E. coli 0157:H7 [ENT/CO] and P. aeruginosa [BTGN]), one yeast (S. cerevisiae) and a fungal organism (A. fumigatus [TY&M]) and performed a dilution series using genomic DNA with estimated copy numbers per millilitre (mL).
The RFU to CFU equation was tested and compared with plating using E. coli 0157:H7 and S. cerevisiae.
Greater efficiencies yield economies of scale
Microarray techniques allow labs to test for class indicator organisms in one sample and one process with same-day results, offering accuracy, speed and streamlined costs.
QuantX yields savings of 20–30% by consolidating all the tests in one sample, limiting associated labour and overheads.
QuantX’s efficiency gains create economies of scale for labs and manufacturers by reducing costs while increasing productivity.
As an example, a lab taking in 100 samples per day with each sample requiring tests for the four class indicator organisms (TAB, BTGN, ENT/COL and TY&M) will need to process a minimum of 400 plates per day.
But, because plate cultures typically require duplicate or triplicate plating to ensure accuracy and statistical significance, 100 samples may require 800–1200 plates.
In addition to the materials this would entail, consider the labour costs of plating 1000 samples and the overhead costs of holding the samples at testing temperatures for 2–6 days.
With QuantX, the same 100 samples would require fewer materials, only a quarter of the labour to process and could be turned around the same day.
These resource and space savings give labs the ability to increase throughput without increasing costs. Such efficiency may be welcome as labs navigate workforce capacity constraints from COVID-19 distancing restrictions.
Providing same-day results eliminates the holding and opportunity costs of recalls to manufacturers as well. Manufacturers no longer have to hold products for days while awaiting results or recall products already shipped, reducing the time and expense of getting products to market.
Culture-independent methods are the future
Labs and manufacturers that primarily test for non-life-threatening micro-organisms have no need to use conventional Petri dish methods when better technologies and methods exist.
Molecular techniques such as QuantX’s multiplexed microarray technology offer an efficient alternative by measuring class indicator organisms more accurately in less time, requiring less labour and fewer materials.




