Introduction
Most active ingredients and mixtures can’t be compressed directly, without wet granulation. Nowadays, the reduction of tablet manufacturing costs is decisive for the industries. Due to this, the pharmaceutical industry and related industries are highly interested in direct compression. With CompactCel ® SIL, a dry binding agent for one-step tableting, it is possible to produce direct compressed tablets containing excipients and a high content of active ingredients (which are normally incompressible) in one step.
The product also enables the user to increase the tablet hardness and improve the friability of tablets at lower compression forces. CompactCel ® SIL provides a granulated and homogeneous compound of Silica and HPC/NaCMC, which acts as a dry binder granulate mixture.
This study will show the influence of various dry binder contents in different formulations to tablet hardness and friability.
Materials and methods
The investigated formulations contain Glucosamine Sulfate 2KCl, Microcrystalline cellulose, Croscar- mellose, Magnesium stearate and a various content of CompactCel ® SIL. The formulation details are listed in table 1. The dry binder compound and excipients were compressed on a Korsch PH 100 DMS, 6-station, rotary press in laboratory scale. The turret speed was increased from 28 RPM (≈ 10,000 tablets per hour) up to 70 RPM (≈ 25,200 tablets per hour). The 400 mg tablets were compressed with a standard round tooling with 10 mm diameter, 5 mm thickness and engraving. For the characterisation the tablets were tested on tablet hardness (according to Ph. Eur. 2.9.8) and friability (according to Ph. Eur. 2.9.7). The used devices and methods are listed in table 2.
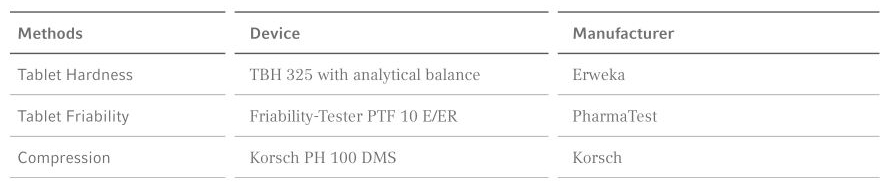
Table 2: Used devices/methods
Results and discussion
The investigated formulations were prepared by simple blending without wet-granulation or pre-compaction of the excipients. For the characterisation, ten tablets of every batch were measured and evaluated. The results are shown in figure 1–4.
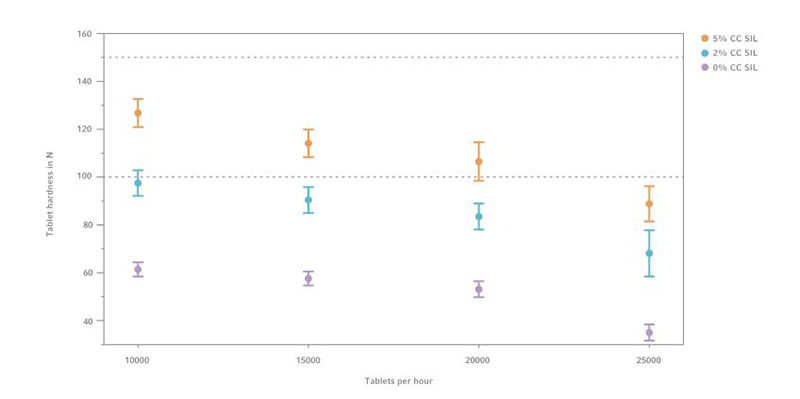
Figure 1: Tablet hardness with =various CC SIL content and 50% GlcN, (n=10)
By increasing the turret speed a significant decrease in the hardness (approximately by half) was observed. This effect can be explained by noticeable dosage inaccuracies due to flow difficulties in the punch-die cavity. Optimal values (between 100–150 N) are shown within the dashed area.
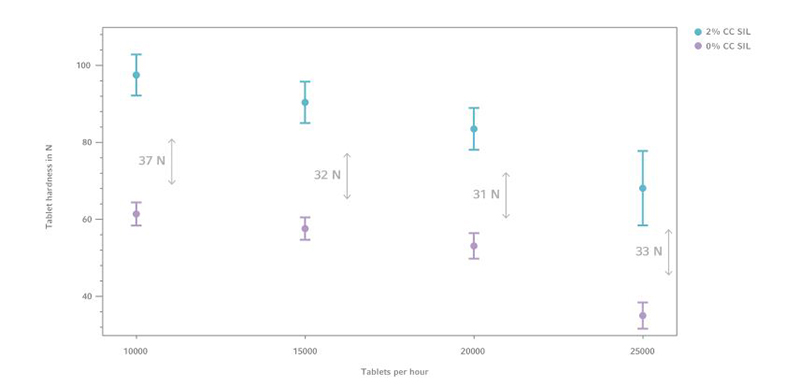
Figure 2: Tablet hardness comparison of 0% and %% CC SIL tablets with 50% GlcN, (n=10)
Formulations with 2% CompactCel ® SIL content show already higher measurable tablet hardness values from 60 N up to 110 N. Comparing the averaged measurement results it can be inferred that CC SIL increases the tablet hardness up to 33 N.
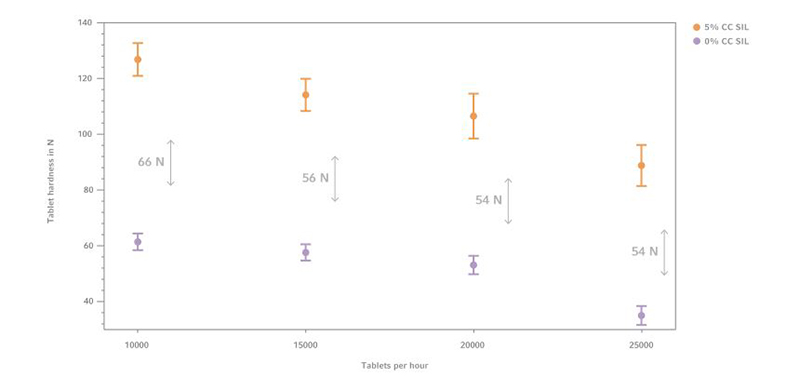
Figure 3: Tablet hardness comparison of 0% and 5% CC SIL with GlcN, (n=10)
Formulations with 5% CompactCel ® SIL content show tablet hardness values from 90 N up to 135 N. Comparing the averaged measurement results it can be inferred that CC SIL increases the tablet hardness up to 57.5 N.
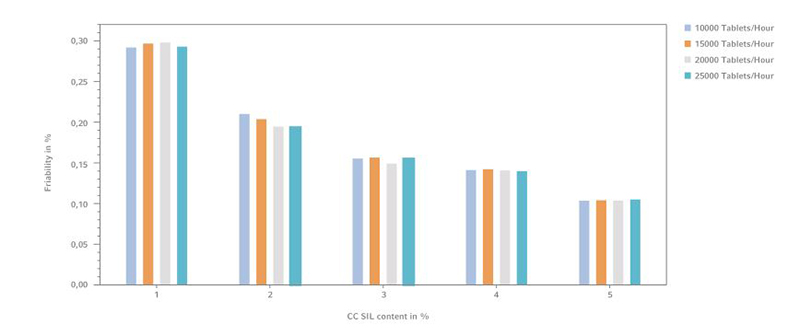
Figure 4: Tablet friability comparison with various CC SIL with GlcN, (n=10)
GlcN tablets without the dry binder compound revealed not evaluable results. This can be explained by broken tablets after the measurement whereby the test failed. As shown in Figure 4 with increasing content of CompactCel ® SIL in the formulations the friability of the tablets decreases. Using already 1% of the dry binding agent in the tablet mass causes friability values lower than 0.3%.
Conclusion
The results of the case study confirm the positive influences of CompactCel ® SIL to directly incom- pressible tablet formulations. The CompactCel ® SIL formulations increase the tablet hardness of glucosamine sulfate tablets up to 2.5 times higher values. It also has a significant influence to tablet friability. Due to the ingredient properties of the dry binder compound it is possible to produce direct compressed tablets in one-step. All in all, CompactCel ® SIL brings decisive benefits for the user. On the one hand, the reduction of manufacturing cost (by saving a work-step) and on the other hand, it is easy to use for employees.




