Bromeyal® is a newly developed and highly characterized bromelain ingredient derived from pineapple stem using a proprietary aqueous extraction and multi-step extraction process.
Even after simulated digestion, the proteolytic enzyme complex found in Bromeyal® (figure 1), provides all the health benefits associated with bromelain without using enteric-coating.
Bromelain is a general name for a family of sulfhydryl proteolytic enzymes obtained from Ananas comosus, the pineapple plant (figure 2).1
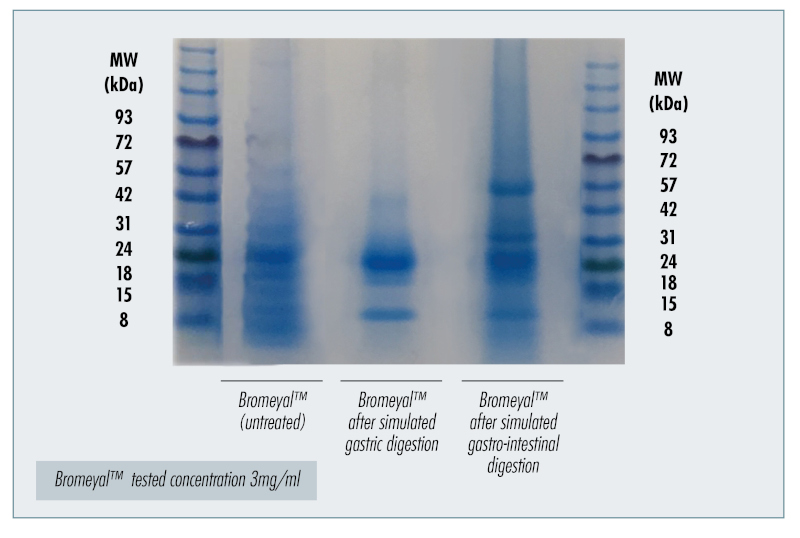
Figure 1: Peptides and protein fractions in Bromeyal® after in vitro simulated digestion
It's typically distinguished as either fruit bromelain or stem bromelain, with the majority of commercially available bromelain derived from the stem.2First identified in pineapple juice in 1891, it has been manufactured on a large scale as nutritional dietary supplement since 1957.
To date, eight proteolytically active constituents have been isolated from bromelain.3 Proteinases are thought to be the active fractions4 and bromelain appears to exert its activity over a pH range of 4.5 to 9.5.5
The biological properties attributed to bromelain include anti-inflammatory, anti- thrombotic, fibrinolytic, and immunomodulatory actions.6
While the interest in bromelain for human use has extended to a wide variety of areas including digestive assistance (when taken with meals), respiratory tract diseases (as a mucolytic), and as a potential adjunct in cancer treatment, the primary focus has been its use in inflammatory conditions such as osteoarthritis and sports injuries.7
Figure 2. Bromelain chemical structure.
A review of clinical trials on bromelain, were either uncontrolled, open-label studies or completed using enzyme combinations that included bromelain.8
Due to its protein structure, bromelain is digested following oral intake and it's generally thought denaturation caused by gastric environment may lead to inactivation and loss of its biological activities (i.e. both enzymatic and anti-inflammatory). Consequently, many dietary supplements available on the market (especially in Europe) are formulated as enteric-coated tablets and capsules.
An in vitro study was recently completed to assess the anti-inflammatory properties of the proprietary bromelain extract, Bromeyal®, in either intact form or following simulated gastrointestinal digestion (hydrolysates) in a variety of human cell lines derived from stomach, intestine and chondrocytes which are all main targets of bromelain activity.9
The results found that Bromeyal®, both in its intact form and after digestion, retains its anti-inflammatory properties. Specifically, the ingredient retains the ability to act directly in lowering interleukin-8 (IL-8) after tumor necrosis alpha (TNF-α) stimulation in the gastrointestinal tract (figure 3).
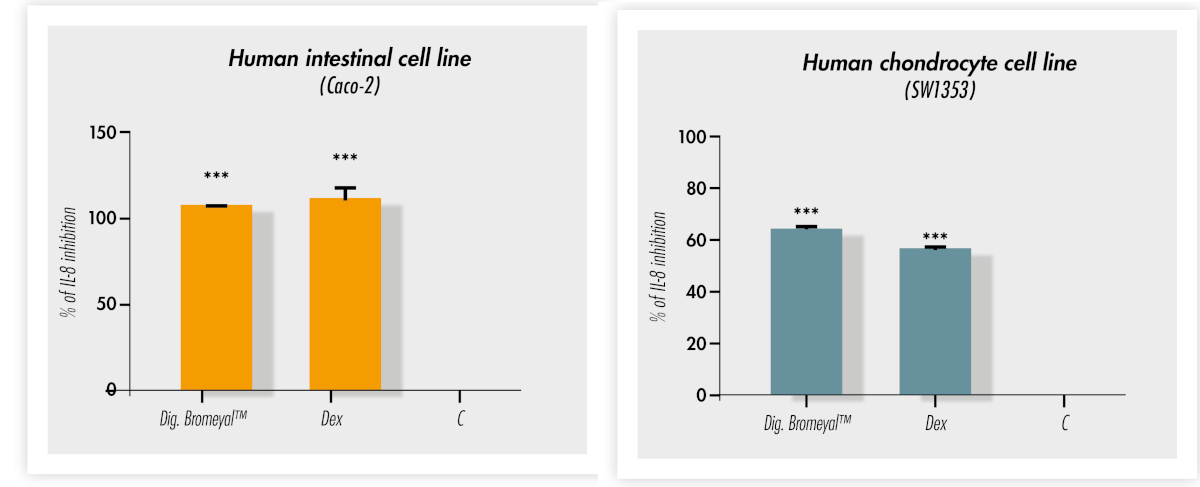
Figure 3: Inhibition of interleukin 8 (IL-8) expression after treatment with digested Bromeyal® in two different cell lines (i.e. chondrocytes and intestinal cells). Legend: Dig. = Bromeyal® after in vitro simulated gastro-intestinal digestion; Dex =dexamethasone used as positive control; C = untreated cells used as negative control. ***P<0.001 Bromeyal® vs control.
This study succeeded in proving the role of Bromeyal® in modulating inflammatory markers in human cell lines derived from stomach, intestine, and chondrocytes, thus corroborating its anti-inflammatory effect in vitro. Of particular note is the maintenance of anti-inflammatory properties following simulated gastrointestinal transit and digestion. This suggests Bromeyal® does not require enteric-coating technologies.
A clinical study to assess the efficacy and safety of Bromeyal® in healthy subjects is scheduled for early 2021.
References
1. Kelly GS. Bromelain: A literature review and discussion of its therapeutic applications. Alt Med Rev 1996;1:243-57.
2. Mauer HR. Bromelain biochemistry, pharmacology and medical use. Cell Molec Life Sci 2001;58:1234-45.
3. Rathnavelu V, Alitheen NB, Sohila S, et al. Potential role of bromelain in clinical and therapeutic applications (Review). Biomed Reports 2016;5:283-8.
4. Murachi R, Yasui M, and Yasuda Y. Purification and physical characterization of stem bromelain. Biochem 1964;3:48-55.
5. Hale LP, Greer PK, Trinh CT, and James CL. Proteinase activity and stability of natural bromlain preparations. Int Immunopharmacol 2005;5:783-93.
6. Pavan R, Shraddha SJ, and Kumar A. Properties and therapeutic application of bromelain: A review. Biotechnol Res International 2012;doi:10.1155/2012/976203.
7. Murray MT and Pizzorno JE. Bromelain. In: Pizzorno JE and Murray MT (eds), Textbook of Natural Medicine. 3 rd Ed. Philadelphia, PA: Elsevier, 2006, pp. 791-5.
8. Brien S, Lewith G, Walker A, et al. Bromelain as a treatment for osteoarthritis: a review of clinical studies. Evidence Base Complementary Alternative Med 2004;1:251-7.
9. Botega R, Persico I, Romano F, Di Lorenzo. Anti-inflammatory properties of a proprietary bromlain extract (Bromeyal®) after in vitro simulated gastrointestinal digestion. Manuscript submitted for publication.
