With around 300 million people known to be affected, asthma is the most common respiratory disease worldwide.1 It’s a chronic inflammatory disease that can be triggered by allergies, by medication such as aspirin or it can arise within the body through yet unclear molecular mechanisms.2
Recurrent episodes of coughing, shortness of breath, wheezing and chest tightness are typical symptoms for asthma.3 Viral infections, cigarette smoking and air pollution can contribute to the worsening of allergic asthma. Regular supplementation with the anti-inflammatory and antioxidant Pycnogenol French maritime pine bark extract has been shown to have several beneficial effects on asthma and allergic reactions.4–13
The effect of Pycnogenol supplementation on allergic asthma management was investigated in several clinical studies.4–6 In a double-blind, placebo-controlled crossover study, the effect of Pycnogenol supplementation for 4 weeks on chronic asthma patients was investigated.4
The patients’ lung function was assessed by analysing the forced expiration volume in 1 second (FEV1), representing the percentage lung volume exhaled in 1 second. In asthmatics, the FEV1 is generally reduced as their breathing is restricted.
After Pycnogenol supplementation, the patients could exhale 70% of their lung volume (compared with 59% on day one) and 63% in response to a placebo (Figure 1). Additionally, the study participants rated their asthma symptoms regarding severity.
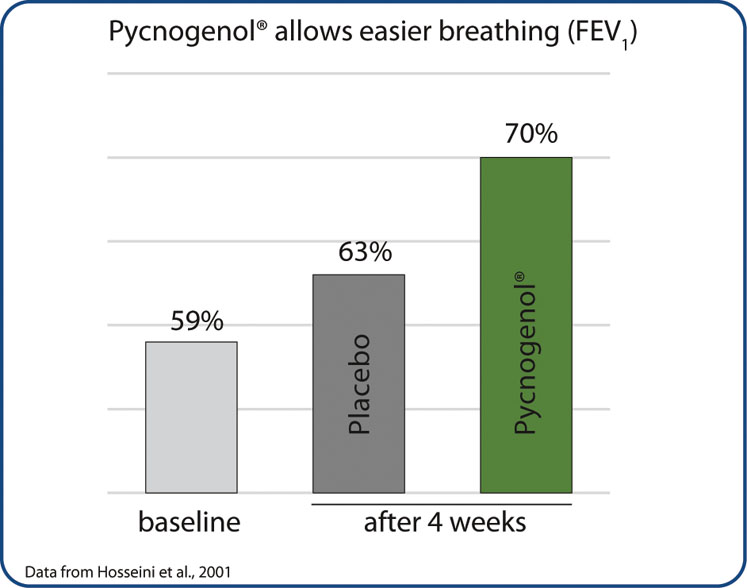
Four weeks of Pycnogenol intake led to a reduction of symptom severity by 20%, whereas it was reduced by only 4.5% in placebo patients. Furthermore, Pycnogenol significantly reduced proinflammatory mediators (leukotrienes) in the blood of patients when compared with both baseline values and the placebo.
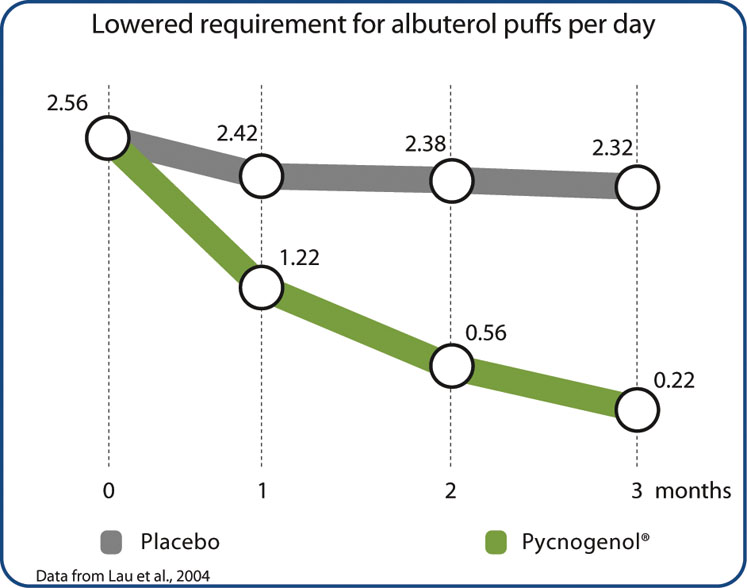
Most asthmatics develop the disease during childhood, often before reaching the age of 5. A double-blind, placebo-controlled study investigated the effect of Pycnogenol on 60 children aged 6–18 with mild to moderate asthma.5
The study showed that their breathing capacity improved significantly after 1 month of treatment with Pycnogenol (as assessed by measuring FEV1). The severity of asthma symptoms as well as leukotriene levels in their urine decreased drastically after 1 month of Pycnogenol supplementation … and further decreased significantly throughout the trial period.
Treatment with a placebo had no significant effect on leukotriene levels or asthma symptoms. The most compelling outcome of the study was the dramatically reduced necessity to use albuterol rescue inhalers; severe asthma attacks occurred much less frequently (Figure 2). After 1 month, eight out of 30 children taking Pycnogenol didn’t require rescue inhalers anymore and 18 children were completely inhaler-free after the 3-month supplementation period.
The effects of Pycnogenol on allergic asthma was investigated in a 6-month study with 79 patients who suffer from dust mite allergy.6 Asthma symptoms, such as dry cough, chest tightness, wheezing, shortness of breath or night-time awakenings significantly improved with Pycnogenol supplementation.
In addition, the patients were able to reduce the use of their salbutamol asthma inhaler per day by half. The authors of the three studies concluded that Pycnogenol is an effective and safe nutritional approach to manage mild-to-moderate chronic asthma and to reduce the need for rescue medication.
Pycnogenol normalises the immune response
Inflammatory processes are a key mechanism in asthma development.2 Pycnogenol’s potent anti-inflammatory activities explain the mechanism regarding its regulation of asthmatic diseases.7,8,14–16
After 5 days of daily intake, a study reported that Pycnogenol significantly prevented the upregulation of the proinflammatory enzymes 5-LOX and COX-2.14 In another study, plasma samples of volunteers after Pycnogenol intake showed a statistically significant inhibition of NF-κB activation by 15.5% and matrix metalloproteinase 9 (MMP-9) release by 25% — two important regulators in the inflammation process.15
The expression of a significant number of proinflammatory genes is governed by NF-κB, such as leukotrienes, cytokines and adhesion molecules. Some of these molecules are known to play an important role in the onset of asthma. The partial inhibition of NF-κB lowers the sensitivity level for triggering an immune response, which will help to prevent an asthmatic attack.
MMP-9 is a connective tissue degrading enzyme, which greatly affects the pulmonary function of asthmatic patients. In a similar study, statistically significant inhibition of inflammatory molecules COX-1 and COX-2 was observed after consuming 300 mg of Pycnogenol.16
These anti-inflammatory effects of Pycnogenol were confirmed in a study with immune cells (macrophages) that are involved in allergic reactions.7 The cells were treated with Pycnogenol, which significantly decreased the elevated production of the inflammation markers IL-1β, IL-6 and MMP-9 in a concentration dependent manner.
Another similar study showed Pycnogenol’s ability to suppress the upregulation of inflammation markers and blocked the activation of an allergy related pathway in the inner layer of lungs (airway epithelial cells) treated with an allergen.8 In addition to its anti-inflammatory action, Pycnogenol has potent antioxidant effects that help to reduce triggers of asthma attacks.17–19
Pycnogenol can relieve the impact of allergies
With 10–30% of the world population suffering from allergic rhinitis, allergies are a very common respiratory problem. Laboratory data and results from clinical trials suggest an amelioration of allergy symptoms after Pycnogenol intake.9–13
In a randomised, double-blind, placebo-controlled trial, researchers found that Pycnogenol supplementation improved allergic rhinitis symptoms from birch and other pollen when the supplementation started at least 5 weeks before the pollen season.9
Eye symptoms decreased by 35% and nasal symptoms by 20.5% compared with a placebo. The number of patients requiring rescue antihistamines was 26% lower in the Pycnogenol group than in the placebo group.
Another study investigated the effects of Pycnogenol intake on wheal and flare evolution after local histamine injection in healthy individuals.10 Pycnogenol supplementation significantly reduced the wheal and flare response by decreasing the redness area around the injection point by 44% or by shortening the time of its complete disappearance by 64%.
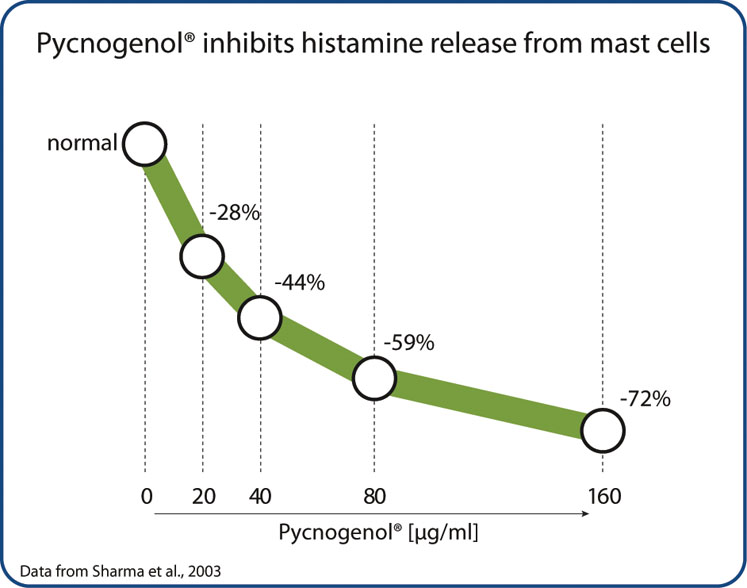
To further understand the mechanism behind these antihistaminic properties, a study investigated Pycnogenol’s effects on immune cells that are involved in allergic reactions. Pycnogenol was found to reduce the release of histamine from the immune cells (mast cells) dose-dependently (Figure 3).11
This might explain Pycnogenol’s preventive action against an immediate immune reaction towards antigens as it occurs in allergies such as hay fever. Interestingly, Pycnogenol was at least as effective at inhibiting histamine release as a widely used antihistaminic medication (sodium cromoglycate). In conclusion, Pycnogenol French maritime pine bark extract is an effective, safe and evidence-based natural solution to manage asthma and allergies.
References
- S.C. Dharmage, et al., “Epidemiology of Asthma in Children and Adults,” Front. Pediatr. 7, 246 (2019).
- M.E. Kuruvilla, et al., “Understanding Asthma Phenotypes, Endotypes and Mechanisms of Disease,” Clin. Rev. Allergy Immunol. 56(2), 219–233 (2019).
- N. Schoettler and M.E. Strek, “Recent Advances in Severe Asthma: From Phenotypes to Personalized Medicine,” Chest. 157(3), 516–528 (2020).
- S. Hosseini, et al., “Pycnogenol in the Management of Asthma,” J. Med. Food 4(4), 201–209 (2001).
- B.H. Lau, et al., “Pycnogenol as an Adjunct in the Management of Childhood Asthma,” J. Asthma 41(8), 825–832 (2004).
- G. Belcaro, et al., “Pycnogenol Improvements in Asthma Management,” Panminerva Med. 53(3), 57–64 (2011).
- I.S. Shin, et al., “Inhibitory Effects of Pycnogenol (French Maritime Pine Bark Extract) on Airway Inflammation in Ovalbumin-Induced Allergic Asthma,” Food Chem. Toxicol. 62, 681–686 (2013).
- Z. Liu, et al., “Pycnogenol Ameliorates Asthmatic Airway Inflammation and Inhibits the Function of Goblet Cells,” DNA and Cell Biology 35, 1–10 (2016).
- D. Wilson, et al., “A Randomized, Double-Blind, Placebo-Controlled Exploratory Study to Evaluate the Potential of Pycnogenol for Improving Allergic Rhinitis Symptoms,” Phytother. Res. 24(8), 1115–1119 (2010).
- G. Belcaro, et al., “Pycnogenol Reduces the Wheal and Flare Response to Histamine in Normal Subjects,” Minerva Biotecnologica 28, 114–119 (2016).
- S.C. Sharma, et al., “Pycnogenol Inhibits the Release of Histamine from Mast Cells,” Phytother. Res. 17(1), 66–69 (2003).
- Y.H. Choi and G.H. Yan, “Pycnogenol Inhibits Immunoglobulin E-Mediated Allergic Response in Mast Cells,” Phytother. Res. 23(12), 1691–1695 (2009).
- A.I.A. Unsal, et al., “Effect of Pycnogenol on an Experimental Rat Model of Allergic Conjunctivitis,” Graefe’s Archive for Clinical and Experimental Ophthalmology 265(7), 1299–1304 (2018).
- R. Canali, et al., “The Anti-Inflammatory Pharmacology of Pycnogenol in Humans Involves COX-2 and 5-LOX mRNA Expression in Leukocytes,” Int. Immunopharmacol. 9(10), 1145–1149 (2009).
- T. Grimm, et al., “Inhibition of NF-kB Activation and MMP-9 Secretion by Plasma of Human Volunteers After Ingestion of Maritime Pine Bark Extract (Pycnogenol),” J. Inflamm. (Lond). 3, 1 (2006).
- A. Schäfer, et al., “Inhibition of COX-1 and COX-2 Activity by Plasma of Human Volunteers After Ingestion of French Maritime Pine Bark Extract (Pycnogenol),” Biomed. Pharmacother. 60(1), 5–9 (2005).
- A. Chovanova, et al., “Effect of Polyphenolic Extract, Pycnogenol, on the Level of 8-Oxoguanine in Children Suffering from Attention Deficit/Hyperactivity Disorder,” Free Radic. Res. 40(9), 1003–1010 (2006).
- S. Devaraj, et al., “Supplementation with a Pine Bark Extract Rich in Polyphenols Increases Plasma Antioxidant Capacity and Alters Plasma Lipoprotein Profile,” Lipids 37(10), 931–934 (2002).
- F. Enseleit, et al., “Effects of Pycnogenol on Endothelial Function in Patients with Stable Coronary Artery Disease: A Double-Blind, Randomized, Placebo-Controlled, Crossover Study,” Eur. Heart J. 33(13), 1589–1597 (2012).
