The results of a bioavailability study on BioTurm, a branded curcumin ingredient from NuAxon Bioscience, were recently published in the Cureus Journal of Medical Science, validating the ingredient’s efficacy over generic curcumin.
BioTurm extract, which is comprised of 45% curcuminoids and four-and-a-half per cent ar-turmerone, offers superior bioavailability without the need for piperine, which is commonly used to enhance curcumin absorption.
This results in cost savings of 25% or more for supplement manufacturers and safer, more effective products for consumers.
Unlike many curcumin ingredients that rely on theoretical data or animal studies, BioTurm was validated in a randomised, three-arm human bioavailability study conducted in healthy adults.
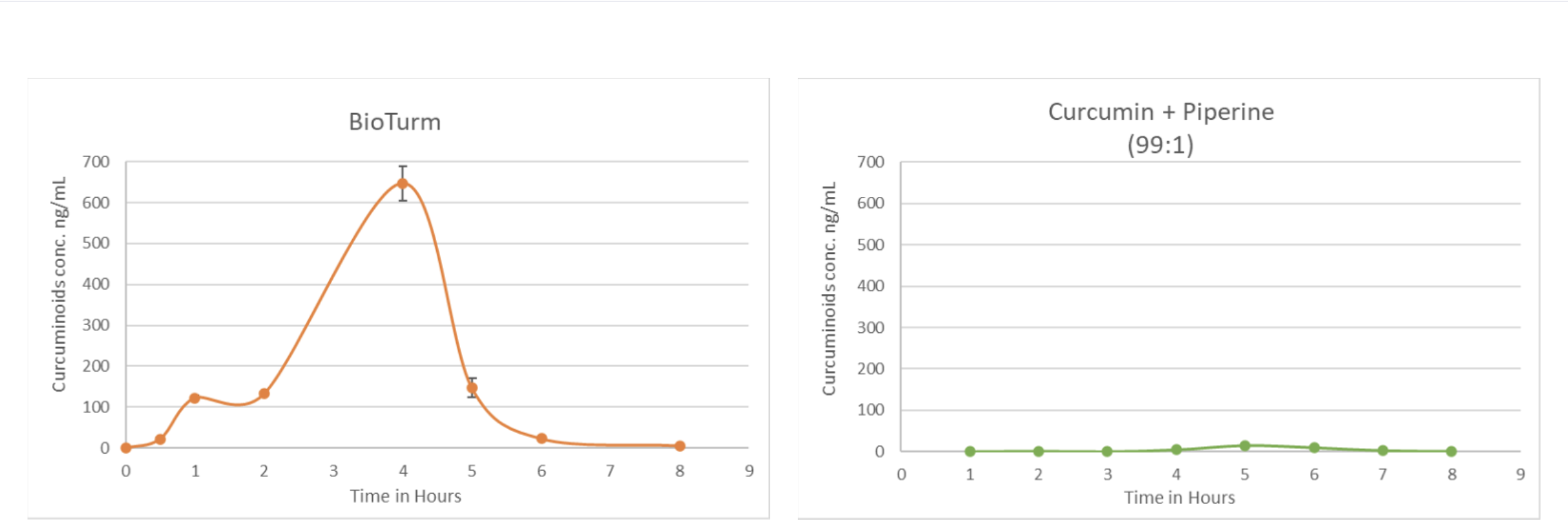
The clinical study compared BioTurm to 95% standard curcumin plus one per cent piperine and 95% standard curcumin plus 10% piperine.
Blood samples were measured during an eight-hour period to track curcuminoid absorption and ar-turmerone bioavailability.
The conclusions include the following:
- BioTurm has 60 times higher bioavailability than the 95% standard curcumin with one per cent piperine
- only the 95% standard curcumin plus ten per cent piperine, a pharmacological dose, matched BioTurm’s bioavailability
- BioTurm provides consistent release for systemic absorption.
BioTurm achieves these results because of its ar-turmerone, a turmeric oil compound that enhances the cellular uptake of curcumin, increases intestinal absorption and provides anti-inflammatory, antioxidant and antimicrobial benefits without synthetic enhancers or altering drug-metabolising enzymes.
Ar-turmerone also acts without causing gastrointestinal discomfort, which can be a common side effect of piperine at higher doses.
"The botanicals industry at large lacks sufficient scientific backing for some claims, impacting credibility broadly," said Devendra Soman, marketing director.
"At NuAxon, we only make claims supported by strong scientific evidence and the publication of our recent clinical study is one way we’re showing our commitment to transparency and authenticity — two values that are core to the company."
