At some point in our lives, we have all experienced the surprising relationship between our brain and our digestive system: the so-called brain–gut axis. During the past few years, a third party has appeared in the axis of communication: the gut microbiota. It has now been shown that by positively influencing the microbiota, certain probiotics can help to modulate gut–brain communication, with positive effects on stress or anxiety.
The digestive tract harbours 70% of the body’s neural cells (the enteric nervous system). The relationship between the brain and the gut has long been recognized and defined as the brain–gut axis: a bidirectional dialogue that co-ordinates brain and gut functions. Based on the fact that the gut harbours around 10 times more bacteria than the number of cells in the body, and 100 times more genes than our own genome, it is not surprising that the microbiota has been found to play a role in gut–brain dialogue. This three-way communication system has been described fairly recently. Changes in host physiology, initiated within the gastrointestinal (GI) tract or by the central nervous system, produce changes in the bacterial composition of the GI tract.
Alternatively, changes in the microbiota, induced by infection or antibiotics for example, or other events such as stress, perturb physiologic inflammation and GI physiology. This can be explained by the fact that a change in GI physiology provides an altered habitat that, in turn, supports a different microbiota. Scientists propose that this cycle could be a basis for maintaining a state of GI dysfunction following microbiotic perturbation; it could also explain the development and persistence of dysbiosis in conditions in which there is a primary disturbance of GI physiology (Figure 1).
In this context, it appears that the use of exogenous microflora, or probiotics, defined as 'live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host', could help to stabilise or normalise the bacterial composition of the GI tract and restore a healthy brain-gut dialogue, with potential psychological and physiological benefits.
A potential for probiotics
Clinical research on probiotics has significantly accelerated during the course of the past 15 years and their modes of action have been more precisely elucidated. Based on recent studies, three possible mechanisms have been put forward to help explain the beneficial effects of probiotics on anxiety and depression that have been shown in human and animal models:
- the competitive exclusion of gut pathogens by probiotics (certain gut pathogens produce substances known to induce anxiety and aggression in animals)
- a decrease in proinflammatory cytokines (a link has been drawn between depression and high levels of certain inflammatory markers)
- direct communication with the central nervous system via vagal sensory fibres, leading to changes in neurotransmitter levels or function.
For example, it has been shown that the strains Lactobacillus helveticus Rosell-52 and Bifidobacterium longum Rosell-175 have the ability to exert anti-inflammatory properties on human intestinal epithelial cells.2 Moreover, the strain Lactobacillus helveticus Rosell-52 was shown to protect the gut microflora against the invasion of pathogenic bacteria; and both strains are known to reduce intestinal permeability thanks to their barrier effect.3 The combination of these actions could lead to a reduction in the inflammation and neuroinflammation caused by stress at the level of the gut mucosa and result in physiological and psychological benefits in stressed subjects. This, combined with the in-depth characterisation of these two particular strains, has prompted scientists to evaluate the benefits of a combination of these two strains on stress and anxiety in volunteers to test this hypothesis.
The first probiotic human study on stress
A double-blind, placebo controlled, randomised study was conducted during a 3-week period with 75 healthy volunteers with symptoms of stress, using a probiotic preparation combining Lactobacillus helveticus Rosell-52 and Bifidobacterium longum Rosell-175 in a stick format (Probio’Stick, Lallemand Health Solutions, Montréal, Canada).4 All the volunteers completed a self-evaluation questionnaire on stress-induced symptoms at the beginning and the end of the 3-week period. After three weeks of daily administration of the probiotic, it appeared that stress-induced gastrointestinal symptoms, nausea and abdominal pain were significantly reduced by 49%, compared with the placebo group (Figure 2). Moreover, the probiotic treatment tended to reduce cardiovascular symptoms such as palpitations.
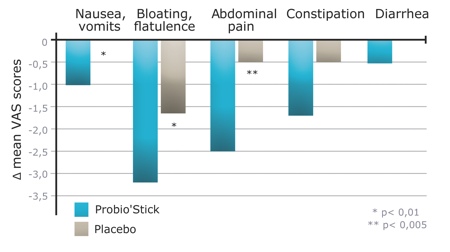
Figure 2: Effects of probiotics on the evolution of various stress-induced gastrointestinal symptoms (variations of VAS score before and after the treatment for each patient group)4
Psychological benefits
Another clinical study was done by Drs Messaoudi and Bisson in Nancy (France) using the same probiotic preparation.5 It assessed the effect of a 1-month administration of the probiotic on anxiety, depression, stress and coping strategies in healthy human volunteers. This study utilised a range of psychological self-assessment tests, commonly used to evaluate anxiolytic drugs, and a biomarker for stress and anxiety (24-hour urinary free cortisol monitoring). The study involved a total of 55 healthy stressed subjects.
The randomised study showed that a one-month daily administration of the probiotic had a beneficial effect on general signs of anxiety and depression (in particular the somatisation, depression and anger-hostility components, as shown by Hopkins Symptom Checklist-90, an instrument used to evaluate a broad range of psychological problems and symptoms of psychopathology) (Figure 3). In addition, these positive results were verified by a decrease of the urinary free cortisol results, showing a reduction of biological stress symptoms. These results show that the probiotic has a beneficial effect on general signs of anxiety and depression, which were supported by the decrease of the 'cortisol' biomarker in the urine.
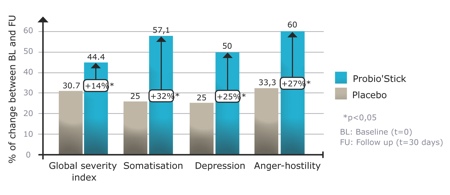
Figure 3: Effects of Probio’Stick on the median HSCL-90 scores after 1 month of treatment5
Anxiolytic-like effect
A preclinical study, based on a rat model of anxiety, which is commonly used to screen anxiolytic agents, was done with 36 male Wistar rats. Receiving 14 days of treatment (Probio’Stick, diazepam or vehicle), the objective was to compare a probiotic with an anxiolytic commonly used to treat anxiety, insomnia, seizures (including status epilepticus) and muscle spasms as a positive control. The study showed a significant difference between the placebo and the anxiolytic, and between the placebo and the probiotic, but not between the probiotic and the anxiolytic (Figure 4). Thus, after two weeks of treatment, the probiotic showed a significant anxiolytic-like effect in this animal model.
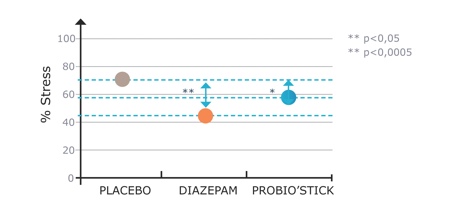
Figure 4: Effect of probiotic on global stress/anxiety score in rats in a conditioned defensive burying test5
Deciphering probiotic modes of action
Subsequently, to understand the effects and mechanisms of action of the probiotic, researchers chose to evaluate its effects using the myocardial infarction (MI) model in rat (to evaluate inflammation, depression and intestinal permeability). Myocardial infarction, generally known as a heart attack, is an ischaemic necrosis of the heart. Four major effects are associated with MI. First, it promotes the release of circulating proinflammatory cytokines, increases intestinal permeability and the propensity for apoptosis (programmed cell death) in the limbic system (the emotional centre of the brain). Finally, it encourages depression — with 65% of patients presenting depressive symptoms after MI and 20% incurring major depression.
Three preclinical studies were conducted with this model.6–8 Altogether, these studies showed that the probiotic both acts on the gut microbiota by decreasing intestinal permeability and on the brain by decreasing apoptosis in the limbic system and depression. The systemic concentration of proinflammatory cytokines was also significantly reduced with the probiotic.
A recent publication by Ait-Belgnaoui et al. looked deeper into the effects of the same probiotic preparation on physiological, cellular and molecular events in the brain and confirmed that the probiotic effects were reflected at brain level.9 This study showed that the probiotic was able to ameliorate chronic stress-related abnormal brain plasticity and reduce neurogenesis.
Clinical and preclinical studies have revealed benefits related to the reduction of stress, anxiety and symptoms of depression
In conclusion, the studies presented confirm that specific probiotics are able to modulate various aspects of the microbiota-gut-brain axis. Even if the mechanisms of action still remain uncertain, probiotics clearly play a key role in the recovery of gut-microbiota disorders, as well as brain and mood disturbances. Clinical and preclinical studies have revealed benefits related to the reduction of stress, anxiety and symptoms of depression. It suggests that probiotics may act on a part of the brain associated with emotions, anxiety and stress, which prevents the activation of the hypothalamic–pituitary–adrenal (HPA) axis. The latest studies further indicate some effects at brain level and anti-inflammatory activity.
Altogether, these benefits indicate that a probiotic preparation could be described as a psychobiotic, as defined by Dinan et al., who concluded that 'to detect psychobiotics, we would favour probiotic strains that preclinically have shown behavioural effects, are delivery vehicles for neuroactive compounds and have a capacity to decrease proinflammatory cytokines and reduce HPA activity.'1
References
1. T.G. Dinan, et al., “Psychobiotics: A Novel Class of Psychotropic,” Biol. Psychiatry 74(10), 720–726 (2013).
2. T.D. Wallace, et al., “Interactions of Lactic Acid Bacteria with Human Intestinal Epithelial Cells: Effects on Cytokine Production,” J. Food Prot. 66, 466–472 (2003).
3. E. Wine, et al., “Strain-Specific Probiotic (Lactobacillus helveticus) Inhibition of Campylobacter jejuni Invasion of Human Intestinal Epithelial Cells,” FEMS Microbiol. Lett. 300, 146–152 (2009).
4. L. Diop, et al., “Probiotic Food Supplement Reduces Stress-Induced Gastrointestinal Symptoms in Volunteers: A Double-Blind, Placebo-Controlled, Randomized Trial,” Nutrition Research 28(1), 1–5 (2008).
5. M. Messaoudi, et al., “Assessment of Psychotropic-Like Properties of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Rats and Human Subjects,” British Journal of Nutrition 105(5), 755–764 (2010).
6. S.A. Girard, et al., “Lactobacillus helveticus and Bifidobacterium longum Taken in Combination Reduce Apoptosis Propensity in the Limbic System After Myocardial Infarction in a Rat Model,” British Journal of Nutrition 102(10), 1420–1425 (2009).
7. J. Arsenaul–Bréard, et al., “Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Reduces Post-Myocardial Infarction Depression Symptoms and Restores Intestinal Permeability in a Rat Model,” British Journal of Nutrition 107(12), 1793–1799 (2011).
8. K. Gilbert, et al., “Attenuation of Post-Myocardial Infarction Depression in Rats by n-3 Fatty Acids or Probiotic Starting After the Onset of Reperfusion,” British Journal of Nutrition 109(1), 50–56 (2012).
9. A. Ait-Belgnaoui, et al., “Probiotic Gut Effect Prevents the Chronic Psychological Stress-Induced Brain Activity Abnormality in Mice,” Neurogastroenterol. Motil. 26(4), 510–520 (2014).

