From the mouth to the gut, our digestive system harbours a rich and complex microflora, the balance of which is essential to our health and well-being. This so-called state of homeostasis is a concept that is increasingly understood and desired by consumers. The benefits of selected probiotics to help maintain this balance and preserve digestive health are also more commonly advocated.
Two types of micro-organisms have been successfully used as probiotics for decades: bacteria, mainly from the Lactobacilli and Bifidobacteria species, and yeast, most particularly Saccharomyces cerevisiae boulardii (the most clinically documented probiotic in the history of medicine). Yeast and bacteria appear to have distinct, albeit complementary, modes of action to help balance the mouth or gut microflora. Based on preclinical and clinical studies conducted on well-characterised strains, there is great potential to be harnessed from a new generation of probiotic solutions combining both yeast and bacteria. Welcome to Probiotic 2.0!
Yeast and bacteria: difference is strength
First, starting with basic microbiology, let’s look at the main characteristics of yeast and bacteria. The most obvious difference is their size: bacteria are approximately ten times smaller than yeast. Their optimal growth conditions also vary: yeasts strive under more acidic conditions than bacteria. Bacteria comprise the major component of the gut microbiota: probiotic bacteria tend to mimic the endogenous flora and adhere to the gut surface, whereas S. boulardii does not adhere to the gut. Yeast is not sensitive to the action of antibiotics, which means it can be administered concomitantly with an antibiotic treatment. Finally, components of the yeast cell walls (glucans, mannan, etc.) are well known for their positive in-host effect on pathogen binding and immune modulation, providing additional benefits.
Complementary modes of action
The benefits of probiotic micro-organisms are linked to their properties and metabolic activity in the host. Their modes of action are increasingly well understood. In the gut, those effects can be divided between three categories.
In the gut lumen: Here, the goal is to deactivate or eliminate potential pathogens to prevent their attachment, colonization and toxic activity. S. boulardii is able to specifically bind to pathogens such as E. coli in the gut lumen, forming aggregates that can easily be eliminated through the gut. Moreover, its ability to inactivate Clostridium difficile toxins A and B is well documented (the toxin-producing bacteria is known to be responsible for 10–25% of antibiotic associated diarrhoea in patients).1
The luminal effect of probiotic bacteria is mainly linked to their metabolism, including the production of certain antagonists (bacteriostatin, H202) or the induction of an acidic environment (lactic acid production). Most pathogens (salmonella, coliforms) are sensitive to low pH levels. For example, Lactobacillus rhamnosus HA-111 and L. fermentum HA-179 have been shown to exert antimicrobial activities against common intestinal pathogens such as E. coli, Salmonella, S. aureus and C. difficile.
At the gut wall: The barrier effect. Probiotic bacteria adhere to the gut epithelium, preventing the adhesion of pathogens through steric hindrance (L. brevis HA-112 and L. plantarum HA-119 show strong adhesion capacity to epithelial cells in vitro, for example). Certain strains act by competitive exclusion, competing with pathogens for nutrients or specific adhesion sites. It has been clearly shown that surface-layer proteins from L. helveticus Rosell-52 prevent the adhesion of E. coli to intestinal cells and improve the gut barrier function.
Other barrier effects include the stimulation of mucus production: L. rhamnosus Rosell-11 has shown the ability to stimulate the production of mucin by epithelial cells, reinforcing epithelial protection. L. helveticus Rosell-52 has also shown the ability to reinforce the gut barrier by strengthening the tight junctions between epithelial cells, leading to a reduced translocation of pathogens.2 Probiotics such as S. boulardii are also able to communicate with enterocytes and help them to preserve normal metabolism (trophic effect). Although not able to adhere to the gut lining, the probiotic yeast also shows a positive effect on the tight junctions of epithelial cells.1
Immunomodulatory effect: Beyond the gut surface, the effect of probiotic yeast and bacteria can still participate to the body’s defences: the increasingly well documented interaction of probiotics with the immune system. For example, L. rhamnosus Rosell-11, Bifidobacterium longum Rosell-175 or B. lactis LAFTI B94 are able to modulate the production of cytokines, the messengers of the immune system. S. boulardii also has a proven anti-inflammatory effect.
Yeast and bacteria: a winning combination
Both yeast and bacteria have different properties and modes of action to account for their clinical benefits in digestive health (Figure 1).1,3 Hence the idea that, by combining both, we could expect to get the best of both worlds and see a synergetic effect!
In a preclinical study by Prof. Bisson, the synergetic effect of combining yeast and bacteria was tested in a rat model of traveller’s diarrhoea (experimental Escherichia coli 055:B5 challenge).4 The rats were fed a placebo or a probiotic preparation (S. boulardii alone, a bacterial combination of Lactobacillus rhamnosus Rosell-11, Lactobacillus helveticus Rosell-52 and Bifidobacterium longum Rosell-175, or both) for 14 days before the pathogen challenge, and for 3 days subsequently.
It was shown that the probiotic yeast and bacteria combination offered better protection against E. coli infection than the probiotic yeast or bacteria mixture separately: symptoms of E. coli infection (fever, weight loss, diarrhoea) were more effectively prevented than with the yeast or bacteria alone (Figure 2).
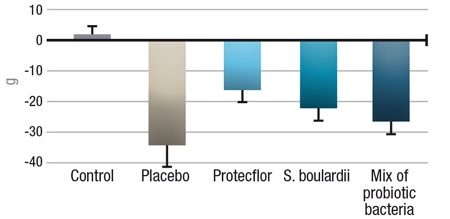
Figure 2: Reduction of weight loss in rats infected with E. coli and treated daily with different probiotic solutions
Moreover, the synergistic effect of yeast and bacteria was also observed at the immunity level, indicating an anti-inflammatory effect: anti-inflammatory cytokines (IL-10 and IL-4) significantly increased, while pro-inflammatory cytokines (IL 1-allpha, IL 1-beta, IL 2, IL 6, GM-CSF, IFN-gamma, TNF-alpha) were lower when compared with the placebo, but also compared with S. boulardii or the bacteria alone in the same model. Other authors suggest that yeasts could positively interact with probiotic bacteria by enhancing their survival and stimulating their growth.5 This positive interaction could be attributed to the production of nutrients such as peptides, amino acids and/or vitamins.
Promising clinical evidence
The combination described earlier associating S. boulardii with Lactobacillus rhamnosus Rosell-11, Lactobacillus helveticus Rosell-52 and Bifiobacterium longum Rosell-175 has been successfully marketed in certain countries, and several post-marketing clinical trials have been published. A first trial, conducted by Grandy, et al. in children hospitalised with acute rotavirus diarrhoea in Bolivia, showed that the combination formula reduced the duration of diarrhoea and therefore the time of rotavirus excretion (Figure 3), reducing the risk of transmission.6
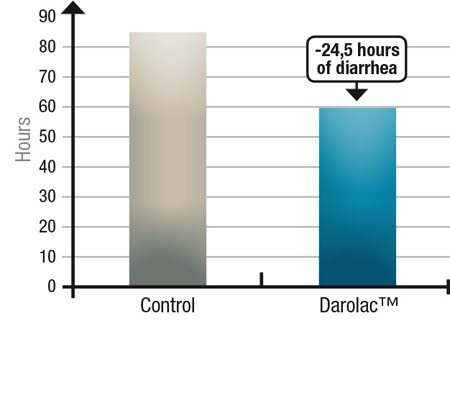
Figure 3: Reduction of diarrhoea duration with a combination probiotic compared with a control in children suffering from rotavirus-induced diarrhoea
Other studies with the same combination examined oral microflora balance (Figure 4):
- the probiotic combination, used as a mouthwash, significantly reduced the salivary count of cariogenic bacteria S. mutans in both children and adults, compared with a placebo, and with a similar efficacy to chlorhexidine and sodium fluoride mouthwashes7,8
- the probiotic effectively reduces dental plaque formation in children, compared with a placebo and traditional antiseptic mouthwashes, and significantly reduced gingivitis risk in children.9,10
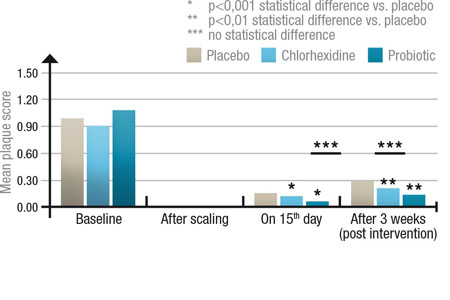
Figure 4: Comparison of dental plaque score between three groups of 30: placebo, chlorohexidine mouthrinse and probiotic mouthrinse; after scaling (0), at the end of the intervention (day 15) and 3 weeks post-treatment
Conclusion
The co-operation between yeast and bacteria is already known and exploited in industry: traditional fermentation processes such as wine making rely on the combined activity of yeast and bacteria, as do traditional health remedies such as kefir or kombucha. Until now, however, probiotic supplements have not really explored the possibilities offered by the complementarity of both types of micro-organisms, probably because of the fact that the selection and industrial production of yeast and bacteria are two different things, and most probiotic producers specialise in either one or the other.
Being an expert in both yeast and bacteria production and applications, Lallemand Health Solutions has the know-how to develop specific probiotic formulations combining pharmaceutical-grade S. boulardii with specific probiotic bacteria. Today, the company has selected a range of well-documented bacterial strains to be combined with S. boulardii to address digestive health, from the mouth to the gut, for a new, holistic approach to digestive health. The Probiotic 2.0 revolution is about to begin!
References
1. T. Kelesidis and C. Pothoulakis, 'Efficacy and Safety of the Probiotic Saccharomyces boulardii for the Prevention and Therapy of Gastrointestinal Disorders,' Ther. Adv. Gastroenterol. 5(2), 111–125 (2012).
2. P.M. Sherman, et al., 'Probiotics Reduce Enterohemorrhagic Escherichia coli O157:H7 and Enteropathogenic E. coli O127:H6-Induced Changes in Polarized T84 Epithelial Cell Monolayers by Reducing Bacterial Adhesion and Cytoskeletal Rearrangements,' Infection and Immunity 73(8), 5183–5188 (2005).
3. L.M. Foster, T.A. Tompkins and W.J. Dahl, 'A Comprehensive Post-Market Review of Studies on a Probiotic Product Containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011,' Beneficial Microbes 2(4), 319–334 (2011).
4. J.F. Bisson and H. Durand, 'Effects of Different Probiotic Formulations On a Traveller’s Diarrhoea Model in Rats,' poster presentation at the 4th Probiotics, Prebiotics and New Foods Congress (16–18 September, Rome, Italy, 2007).
5. Y. Katakura, et al., 'Lactic Acid Bacteria Display on the Cell Surface Cytosolic Proteins that Recognize Yeast Mannan,' Appl. Microbiol. Biotechnol. 86(1), 319–326 (2010).
6. G. Grandy, et al., 'Probiotics in the Treatment of Acute Rotavirus Diarrhoea. A Randomized, Double-Blind, Controlled Trial Using Two Different Probiotic Preparations in Bolivian Children,' BMC Infect. Dis. 10, 253 (2010).
7. G. Jindal, et al., 'A Comparative Evaluation of Probiotics on Salivary Mutans streptococci Counts in Indian Children,' Eur. Arch. Paediatr. Dent. 12, 211–215 (2011).
8. M. Jothika, P.P. Vanajassun and B. Someshwar, 'Effectiveness of Probiotic, Chlorhexidine and Fluoride Mouthwash Against Streptococcus mutans: A Randomized, Single-Blind, In Vivo Study,' J. Int. Soc. Prev. Community Dent. 5(Suppl. 1), 44–48 (2015).
9. P.K. Thakkar, et al., 'Effect of Probiotic Mouthrinse on Dental Plaque Accumulation: A Randomized Controlled Trial,' Dent. Med. Res. 1, 7–12 (2013).
10. S. Purunaik, H.M. Thippeswamy and S.S. Chavan, 'To Evaluate the Effect of Probiotic Mouthrinse on Plaque and Gingivitis among 15–16 Year Old School Children of Mysore City, India: A Randomized Controlled Trial,' Global Journal of Medical Research: J. Dentistry and Otolaryngology 14(4), Version 1.0 (2014).





