A growing number of well conducted studies are linking natural plant-based phytochemicals with a lower risk of chronic illnesses such as dementia, high cholesterol, arthritis, skin ageing and macular degeneration. The evidence for their impact on cancer incidence and relapse is also becoming increasingly convincing as more clinical research is emerging.
There are now several large prospective cohort studies that demonstrate that people who eat diets rich in polyphenols have a lower risk of cancer. Links are especially strong for breast, pancreas, colorectal, oesophagus, ovary and prostate cancers. For example, a large meta-analysis recorded a significant relationship between the incidence of breast cancer with a higher consumption of carotenoid-rich foods such as leafy green vegetables, carrots, broccoli and asparagus.
Although there is still a lack of evidence from randomised trials, the sheer number of cohort studies with the same conclusions are convincing
Another study linked the regular long-term intake of antioxidant-rich foods — such as herbs, spices, tea, fruit and vegetables — with a lower incidence of pancreatic cancer, and a health professional study linked colourful fruits, berries and tomatoes with a lower risk of prostate cancer. A recent study from South Carolina linked flavonoid-rich foods, such as beans, pulses and legumes, with a lower risk of aggressive prostate cancer. Although there is still a lack of evidence from randomised trials, the sheer number of cohort studies with the same conclusions are certainly convincing.
Benefits of polyphenol-rich foods after cancer
The evidence for the benefits of polyphenol-rich healthy foods does not stop after a diagnosis. A study involving 1490 breast cancer survivors from the USA showed that those regularly taking higher than the government recommended five daily fruits and vegetable a day had a third lower breast cancer recurrence risk, especially if combined with regular physical activity. Another study showed that women with breast cancer with high lignan levels in their bloodstream — reflecting a good intake of foods such as legumes, cereals, cruciferous vegetables, coffee and soy — had the lowest risk of death. The Shanghai Breast Cancer Survival Study of 5042 Chinese women patients showed that those with the highest intake of isoflavones and flavanone-rich foods such as soya had a 29% lower risk of death. Another major cohort study showed that regular green tea consumption was associated with a lower breast cancer recurrence.
An Australian study involving individuals who had already been treated for the common types of skin cancer occurring later in life (basal cell carcinoma or squamous cell carcinoma) showed that those who had a high lutein zeaxanthin (leafy green vegetables) intake had a lower rate of new cancer formation. Men with prostate cancer randomised to a lifestyle programme, including healthy eating, had a slower rate of prostate-specific antigen (PSA) progression. A Phase II study reported that the PSA doubling time (PSAdt) was significantly prolonged in men given 200 mL of pomegranate juice a day. A number of other studies evaluating the impact polyphenols after cancer are currently under way. The largest and probably most comprehensive of these is the UK’s DietCompLyf prospective trial, involving 3159 women treated for breast cancer.
The anticancer mechanisms of phytochemicals
The most quoted anticancer mechanism of phytochemicals is via an antioxidant pathway, either by inducing antioxidant enzymes such as superoxide dismutase, catalase and glutathione or by absorbing free radicals directly. Either way, their regular consumption protects the DNA from oxidative damage caused by ingested or environmental carcinogens.
A good example of this was demonstrated in a laboratory study involving cancer cell lines. Exposed to a known household carcinogen (triclocarban), they rapidly mutate into cancer cells. By contrast, adding curcumin to the culture feed significantly reduced the amount and rate of carcinogenesis. Another study conducted by the Food Safety Consortium at the University of Arkansas showed that marinating meat in rosemary and thyme reduced the heterocyclic amine (HCA) content by 87%. This and other carcinogens produced by grilling at high temperatures are known to increase the risk of prostate cancer in human and laboratory studies.
In addition to their antioxidant absorption capacity, certain phytochemicals are able to bind to the oestrogen receptor
In addition to their antioxidant absorption capacity, certain phytochemicals are able to bind to the oestrogen receptor. These phytoestrogenic compounds, most notably isoflavones and lignans, are found in soy products, legumes, pulses and cruciferous vegetables, and weakly bind to the oestrogen receptor without stimulating cell proliferation; yet, at the same time, they block the binding of more harmful oestrogens This includes those produced by your own body or those ingested or absorbed such as xenoestrogens and metaloestrogens. In men, they have been shown to affect 5-alpha reductase-lowering endogenous testosterone levels.
An increasing number of research laboratories are conducting experiments that are revealing significant beneficial effects of polyphenols on the fundamental mechanism of cancer growth, invasion and metastasis, including the following:
- Pomegranate: With a high concentration of ellagic acid, pomegranate has been shown to directly inhibit cell growth and induce apoptosis in androgen-sensitive and aggressive human prostate cancer cells. It has also been reported to inhibit processes involved in cancer metastasis in a study using oestrogen-sensitive and resistant breast cancer cell lines, increasing markers of cell adhesion and migration in cancer but not normal cells; and in another, it inhibited a chemokine that attracts breast cancer cells to the bone and the expression of a gene that is important in epithelial-to-mesenchymal transitions.
- Green tea: Rich in epigallocatechin gallate (EGCG), green tea blocks ornithine decarboxylase, an enzyme that signals cells to proliferate faster and bypass apoptosis. Green tea, in laboratory studies, has demonstrated the significant reduction of several factors that promote breast and prostate cancer cell growth, dedifferentiation and angiogenesis.
- Broccoli: Rich in isothiocyanate (ITC) and its metabolite sulforaphane, broccoli has been found to inhibit growth and promote the apoptosis of cancer cells. One study found that broccoli sparks numerous genetic changes, activating cancer suppressor genes and switching off promotion genes. Broccoli induces the antioxidant enzymes glutathione S-transferases, which explains why it is particularly beneficial in the 50% of the population carrying a mutated glutathione gene (GSTM1).
- Curcumin: Giving turmeric its yellow colour, curcumin slows cancer cell growth by blocking the cell cycle, increasing the rate of apoptosis and preventing the invasion and migration of cells. Research conducted at the University of Michigan also found that turmeric helped to halt the growth of stem cells that give rise to breast cancer without harming normal breast cells.
Concentrating foods into supplements to enhance anticancer effects
If certain foods have anticancer effects, then it is logical to extrapolate that concentrating them into a pill may help to correct for poor diets or further enhance the benefits of already adequate ones. Cancer survivors are attracted to the potential health benefits of food supplements, with more than 70% reporting regular intake. There are two main categories of commercially available supplements: the first involves chemicals extracted from food or made synthetically; the second involves concentrating a whole polyphenol-rich food.
The majority of studies, to date, have evaluated extracted chemicals such as vitamins and minerals. Some have shown a benefit. For example, a recent meta-analysis showed that women who took supplements providing an average daily intake of vitamin C (more than 100mg) had a reduced risk of breast cancer relapse. The SU.VI.MAX Study randomised French adults to a single daily capsule of ascorbic acid, vitamin E, beta-carotene, selenium and zinc, or a placebo, and found no reduction in mortality or cancer-specific mortality overall — although a further analysis in men found a reduction in the risk of prostate cancer. The authors postulated this difference in sex was related to French men having a lower baseline micronutrient status. For the same reason, a major trial of selenium and vitamin supplements in poor regions of China demonstrated reduced risks of oesophageal cancer as, at the time, this population was known to have widespread micronutrient deficiencies.
Unfortunately, most other studies have showed no benefit or were actually linked to an increased risk of cancer. For example, the CARET study found that beta-carotene and retinol increased the risk of lung cancer. Likewise, the ATBC study found that alpha vitamin E and beta-carotene increased lung cancer risk but, in a subsequent analysis, men with pre-intervention low plasma levels of beta-carotene had a lower prostate cancer risk following supplementation; yet, those with high levels had a higher risk, particularly in smokers.

This U-shaped distribution of risk was also observed in the EPIC study, with those with diets deficient in folate and those taking the highest folate intake both having a higher cancer risk. Two other Scandinavian studies demonstrated a higher cancer risk following vitamin B supplementation intake. A double-blind, randomised trial involving men with progressive prostate cancer showed no benefit for a supplement containing salicylate alone versus salicylate plus vitamin C, copper and manganese gluconate.
In the health professional follow-up study, men who took more than 100mg/day of zinc for long durations were more than twice as likely to develop advanced prostate cancer compared with controls. The SELECT study showed an increased prostate cancer incidence with vitamin E and selenium. A study from Australia showed that individuals who took beta-carotene and vitamin E supplements had a higher rate of new skin cancer formation. These data have prompted organizations such as the National Cancer Institute to issue statements saying that long-term vitamin and mineral use should be discouraged unless correcting a known deficiency, and that micronutrient testing is now becoming more widely available (cancernet.co.uk).
Despite some initial encouragement from smaller evaluations of extracted phytochemicals, studies involving Saw Palmetto or Genistein — given on their own in more scientifically robust analyses — have not demonstrated a benefit for either prostate cancer or benign prostatic hypertophy. Likewise, despite the initial enthusiasm for lycopene-containing foods from cohort observations, the two most recent trials of lycopene extracts found no difference in PSA progression, nor were there links to the reduction in the risks of breast cancer with regular intake.
More recently, scientific attention has turned towards the evaluation of concentrated whole foods, particularly those rich in polyphenols
More recently, scientific attention has turned towards the evaluation of concentrated whole foods, particularly those rich in polyphenols and other healthy phytochemicals (herbs, spices, green vegetables, teas and colourful fruits). A study of pomegranate seed extract from Johns Hopkins, which gave men a pill containing pomegranate seed extract, found a reduction in PSA progression rate. The Mayo Clinic found that green tea extract decreased the abnormal white cell count in 30% of patients with chronic leukaemia and a study from Louisiana University reported a significant reduction in the levels of several growth factors that promote cancer as well as a reduction in PSA among participants.
In the VITamins and Lifestyle (VITAL) cohort study, grape seed extract was linked to a lower risk of prostate cancer following regular intake. In a small trial, a dietary supplement containing isoflavones and a natural antioxidant-rich supplement was shown to have a reduction in cancer risk. A series of studies with polyphenol-rich foods, given either on their own or in combination, has been registered with the National Cancer Institute, USA, and are under way or recently completed.
The Pomi-T study
The largest of these trials in the UK is the National Cancer Research Network Pomi-T study. It combined four different food types (berry, vegetable, spice and leaf) to provide a wide spectrum of polyphenol nutrients, while at the same time avoiding the over-consumption of one particular type. It involved more than 200 men with localised prostate cancer, managed with active surveillance (AS) or watchful waiting (WW), experiencing a PSA relapse following initial radical interventions. They were randomised to receive an oral capsule containing a blend of pure and whole pomegranate, green tea, broccoli and turmeric, or a placebo, for six months.
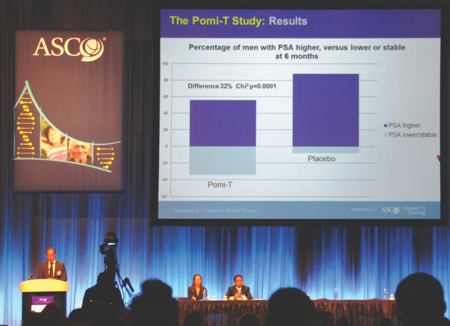
The author presenting the results of the Pomi-T study at the American Society of Clinical Oncology conference
This non-commercial trial was sponsored by the charity Prostate Action, approved by the National Ethics Committee and was peer reviewed by the National Cancer Research Institute (NCRI) Complementary Therapies Research Committee. The randomisation process was outsourced and the trial methodology, collection and storage of data were verified and independently audited by an external agency to ensure adherence to European good clinical practice (GCP). At the end of the trial, the data were externally audited for a second time to ensure there were no data inconsistencies or deviations from the trial design, before the database was sealed and sent for blinded analysis by the statisticians at Cranfield University. The UK manufacturers of the food supplement adhered to good manufacturing practice (GMP) guidelines and performed in-house analyses for authenticity and purity.
The study completed recruitment 10 months ahead of schedule and found a highly statistically significant 63% reduction in the median PSA progression rate compared with the placebo (Figure 1). It was well tolerated, apart from some mild loosening of the bowels in 10% of men, and there was no effect on testosterone levels. At the request of the peer reviewers, a further analysis of tumour size, seen using MRI images, was found to correlate with PSA changes.
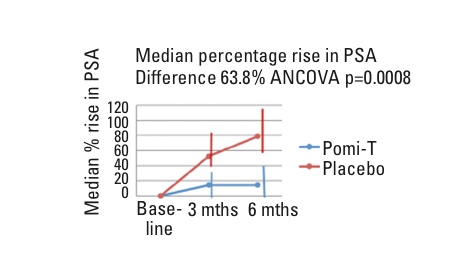
Figure 1: Median percentage rise in PSA (difference = 63.8%; ANCOVA p = 0.0008)
The subsequent presentation of the results at the American Society of Clinical Oncology conference (Chicago, USA) received immediate acclaim from the 28,000 attending oncologists and worldwide recognition from academic institutions and patient advocacy groups. It was seen as a major breakthrough for nutritional research groups, which, for years, had been waiting for adequately powered, well-designed randomised controlled trials in this area.
A further analysis, presented at the UK’s National Cancer Research Institute Conference (Liverpool, UK) has now revealed an extra benefit that could save millions of healthcare dollars and spare numerous men the toxicity of medical treatments. In addition to PSA, the intake of Pomi-T had a significant impact on the management decisions of men in the trial: 5.9% on Pomi-T as opposed to 23.4% in the placebo group opted to leave AS or WW for more aggressive management pathways.
This difference of 17.5% was highly statistically significant (Chi2 p=0.014, Figure 2). Some of these men had radiotherapy or surgery, but most had medical castration, which can cause unpleasant effects such as depression, hot flushes, weight gain, osteoporosis and erectile dysfunction. The costs of interventions can include radiotherapy and surgery, the management of troublesome symptoms and the price of the drugs to induce castration (up to £1000 a year), which some men can stay on for many years. It costs just £170 a year for Pomi-T.
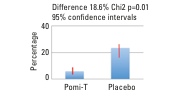
Figure 2: Men remaining on surveillance
Despite these findings, Pomi-T is still classed as a food supplement, according to MHRA and EU commission guidelines, so can never become a recognised medicinal product or find its way into routine management; but at least this and other food supplement studies are providing data for individuals to make more informed self help lifestyle choices. Following the success of this study, the research trials committee has been approached by the PROVENT trials committee to include Pomi-T in the next national prostate cancer prevention study. This study will also recruit men with a higher genetic risk of prostate cancer.
Further trials are being designed in New York and South Africa with early prostate cancer and in the UK involving men with prostate cancer already on androgen-deprivation therapy. Funding is being sought to design studies to evaluate potential benefits for individuals with skin, colorectal and bladder cancer. In the meantime, a trial is passing through the regulatory process to investigate whether the natural anti-inflammatory properties of these ingredients could help joint pains after breast cancer.
In conclusion, the role of polyphenols in the cancer process is becoming more convincing as more evidence is emerging from well-conducted clinical trials. Patients with cancer should be counselled on the benefits of eating polyphenol-rich foods after their initial treatments. Following the success of the Pomi-T study, researchers should hopefully be encouraged to design further trials involving polyphenol-rich food supplements as a low risk, low cost lifestyle strategy to potentially slow progression and reduce relapse rates.
The author
Professor Robert Thomas is Consultant Oncologist in the Department of Oncology at Addenbrooke’s Hospital in Cambridge, UK.
A comprehensive bibliography is available from the author to support the claims made in this article. Email: rt@cancernet.co.uk




